Small-worldness favours network inference in synthetic neural networks
- PMID: 32042036
- PMCID: PMC7010800
- DOI: 10.1038/s41598-020-59198-7
Small-worldness favours network inference in synthetic neural networks
Abstract
A main goal in the analysis of a complex system is to infer its underlying network structure from time-series observations of its behaviour. The inference process is often done by using bi-variate similarity measures, such as the cross-correlation (CC) or mutual information (MI), however, the main factors favouring or hindering its success are still puzzling. Here, we use synthetic neuron models in order to reveal the main topological properties that frustrate or facilitate inferring the underlying network from CC measurements. Specifically, we use pulse-coupled Izhikevich neurons connected as in the Caenorhabditis elegans neural networks as well as in networks with similar randomness and small-worldness. We analyse the effectiveness and robustness of the inference process under different observations and collective dynamics, contrasting the results obtained from using membrane potentials and inter-spike interval time-series. We find that overall, small-worldness favours network inference and degree heterogeneity hinders it. In particular, success rates in C. elegans networks - that combine small-world properties with degree heterogeneity - are closer to success rates in Erdös-Rényi network models rather than those in Watts-Strogatz network models. These results are relevant to understand better the relationship between topological properties and function in different neural networks.
Conflict of interest statement
The authors declare no competing interests.
Figures


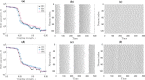
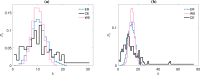
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

