Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories
- PMID: 32042144
- PMCID: PMC7115869
- DOI: 10.1038/s41583-019-0260-z
Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories
Abstract
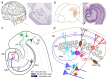
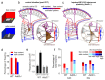
The dentate gyrus (DG) has a key role in hippocampal memory formation. Intriguingly, DG lesions impair many, but not all, hippocampus-dependent mnemonic functions, indicating that the rest of the hippocampus (CA1-CA3) can operate autonomously under certain conditions. An extensive body of theoretical work has proposed how the architectural elements and various cell types of the DG may underlie its function in cognition. Recent studies recorded and manipulated the activity of different neuron types in the DG during memory tasks and have provided exciting new insights into the mechanisms of DG computational processes, particularly for the encoding, retrieval and discrimination of similar memories. Here, we review these DG-dependent mnemonic functions in light of the new findings and explore mechanistic links between the cellular and network properties of, and the computations performed by, the DG.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Schacter DL, Tulving E. Memory systems 1994. MIT Press; 1994.
-
- Eichenbaum H. The cognitive neuroscience of memory: an introduction. Oxford University Press; 2012.
-
- Squire LR. Mechanisms of memory. Science. 1986;232:1612–1619. - PubMed
-
- Tonegawa S, Morrissey MD, Kitamura T. The role of engram cells in the systems consolidation of memory. Nature Reviews Neuroscience. 2018;19:485–498. - PubMed
-
- Corkin S. What’s new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3:153–160. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

