Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory
- PMID: 32042174
- PMCID: PMC7306053
- DOI: 10.1038/s41593-020-0588-8
Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory
Abstract
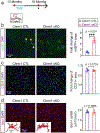
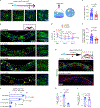
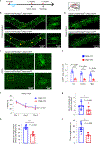
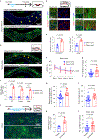
Cognitive decline remains an unaddressed problem for the elderly. We show that myelination is highly active in young mice and greatly inhibited in aged mice, coinciding with spatial memory deficits. Inhibiting myelination by deletion of Olig2 in oligodendrocyte precursor cells impairs spatial memory in young mice, while enhancing myelination by deleting the muscarinic acetylcholine receptor 1 in oligodendrocyte precursor cells, or promoting oligodendroglial differentiation and myelination via clemastine treatment, rescues spatial memory decline during aging.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
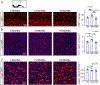


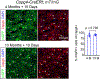

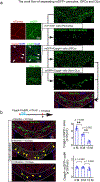

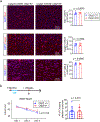
Comment in
-
Myelin makes memories.Nat Neurosci. 2020 Apr;23(4):469-470. doi: 10.1038/s41593-020-0606-x. Nat Neurosci. 2020. PMID: 32094969 Free PMC article.
References
-
- Gazzaley A, Cooney JW, Rissman J & D’Esposito M Top-down suppression deficit underlies working memory impairment in normal aging. Nat. Neurosci 8, 1298–1300 (2005). - PubMed
-
- Garde E et al. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 356, 628–634 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

