Preservation of a remote fear memory requires new myelin formation
- PMID: 32042175
- PMCID: PMC7213814
- DOI: 10.1038/s41593-019-0582-1
Preservation of a remote fear memory requires new myelin formation
Abstract
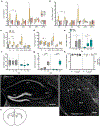
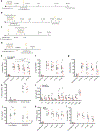
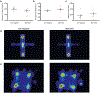
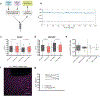
Experience-dependent myelination is hypothesized to shape neural circuit function and subsequent behavioral output. Using a contextual fear memory task in mice, we demonstrate that fear learning induces oligodendrocyte precursor cells to proliferate and differentiate into myelinating oligodendrocytes in the medial prefrontal cortex. Transgenic animals that cannot form new myelin exhibit deficient remote, but not recent, fear memory recall. Recording population calcium dynamics by fiber photometry, we observe that the neuronal response to conditioned context cues evolves over time in the medial prefrontal cortex, but not in animals that cannot form new myelin. Finally, we demonstrate that pharmacological induction of new myelin formation with clemastine fumarate improves remote memory recall and promotes fear generalization. Thus, bidirectional manipulation of myelin plasticity functionally affects behavior and neurophysiology, which suggests that neural activity during fear learning instructs the formation of new myelin, which in turn supports the consolidation and/or retrieval of remote fear memories.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures










Comment in
-
Myelin makes memories.Nat Neurosci. 2020 Apr;23(4):469-470. doi: 10.1038/s41593-020-0606-x. Nat Neurosci. 2020. PMID: 32094969 Free PMC article.
-
Commentary: Preservation of a remote fear memory requires new myelin formation.Front Neural Circuits. 2020 May 19;14:28. doi: 10.3389/fncir.2020.00028. eCollection 2020. Front Neural Circuits. 2020. PMID: 32508599 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

