Using human genetics to understand the disease impacts of testosterone in men and women
- PMID: 32042192
- PMCID: PMC7025895
- DOI: 10.1038/s41591-020-0751-5
Using human genetics to understand the disease impacts of testosterone in men and women
Abstract
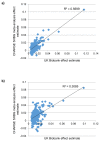
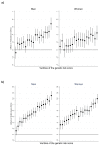
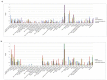
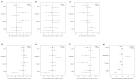
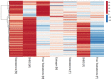
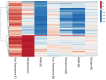
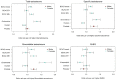
Testosterone supplementation is commonly used for its effects on sexual function, bone health and body composition, yet its effects on disease outcomes are unknown. To better understand this, we identified genetic determinants of testosterone levels and related sex hormone traits in 425,097 UK Biobank study participants. Using 2,571 genome-wide significant associations, we demonstrate that the genetic determinants of testosterone levels are substantially different between sexes and that genetically higher testosterone is harmful for metabolic diseases in women but beneficial in men. For example, a genetically determined 1 s.d. higher testosterone increases the risks of type 2 diabetes (odds ratio (OR) = 1.37 (95% confidence interval (95% CI): 1.22-1.53)) and polycystic ovary syndrome (OR = 1.51 (95% CI: 1.33-1.72)) in women, but reduces type 2 diabetes risk in men (OR = 0.86 (95% CI: 0.76-0.98)). We also show adverse effects of higher testosterone on breast and endometrial cancers in women and prostate cancer in men. Our findings provide insights into the disease impacts of testosterone and highlight the importance of sex-specific genetic analyses.
Conflict of interest statement
The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. M.I.M has served on advisory panels for Pfizer, NovoNordisk and Zoe Global, has received honoraria from Merck, Pfizer, Novo Nordisk and Eli Lilly, and research funding from Abbvie, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, NovoNordisk, Pfizer, Roche, Sanofi Aventis, Servier, and Takeda. As of June 2019, M.I.M is an employee of Genentech, and a holder of Roche stock. T.M.F. holds a MRC CASE studentship with GSK; has consulted for Sanofi, Servier and Boerhinger Ingelheim.
Figures
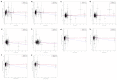
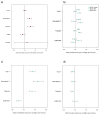

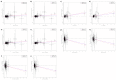
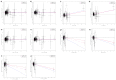

Comment in
-
Genetic analysis reveals role of testosterone levels in human disease.Nat Rev Endocrinol. 2020 Apr;16(4):195. doi: 10.1038/s41574-020-0338-8. Nat Rev Endocrinol. 2020. PMID: 32080496 No abstract available.
References
-
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–99. - PubMed
-
- Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199:548–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

