Coumarin-piperazine derivatives as biologically active compounds
- PMID: 32042262
- PMCID: PMC7000312
- DOI: 10.1016/j.jsps.2019.11.025
Coumarin-piperazine derivatives as biologically active compounds
Abstract
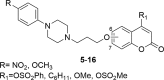
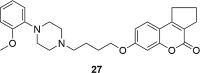
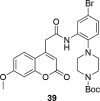
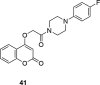
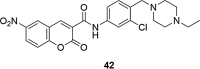
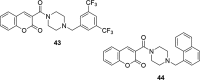
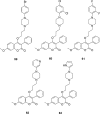
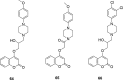
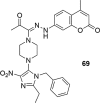
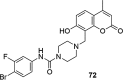
A number of psychiatric disorders, including anxiety, schizophrenia, Parkinson's disease, depression and others CNS diseases are known to induce defects in the function of neural pathways sustained by the neurotransmitters, like dopamine and serotonin. N-arylpiperazine moiety is important for CNS-activity, particularly for serotonergic and dopaminergic activity. In the scientific literature there are many examples of coumarin-piperazine derivatives, particularly with arylpiperazines linked to a coumarin system via an alkyl liner, which can modulate serotonin, dopamine and adrenergic receptors. Numerous studies have revealed that the inclusion of a piperazine moiety could occasionally provide unexpected improvements in the bioactivity of various biologically active compounds. The piperazine analogs have been shown to have a potent antimicrobial activity and they can also act as BACE-1 inhibitors. On the other hand, arylpiperazines linked to coumarin derivatives have been shown to have antiproliferative activity against leukemia, lung, colon, breast, and prostate tumors. Recently, it has been reported that coumarin-piperazine derivatives exhibit a Fneuroprotective effect by their antioxidant and anti-inflammatory activities and they also show activity as acetylcholinesterase inhibitors and antifilarial activity. In this work we provide a summary of the latest advances in coumarin-related chemistry relevant for biological activity.
Keywords: Arylpiperazinyl moiety; Biological activity; Coumarin.
© 2019 The Author.
Conflict of interest statement
Author declares no conflict of interest.
Figures













Similar articles
-
Therapeutic Journey and Recent Advances in the Synthesis of Coumarin Derivatives.Mini Rev Med Chem. 2022;22(9):1314-1330. doi: 10.2174/1389557521666211116120823. Mini Rev Med Chem. 2022. PMID: 34784861 Review.
-
Design, synthesis, biological evaluation, and molecular modeling of coumarin-piperazine derivatives as acetylcholinesterase inhibitors.Arch Pharm (Weinheim). 2013 Nov;346(11):793-804. doi: 10.1002/ardp.201300242. Epub 2013 Oct 8. Arch Pharm (Weinheim). 2013. PMID: 24591157
-
Characterization of potent and selective antagonists at postsynaptic 5-HT1A receptors in a series of N4-substituted arylpiperazines.J Med Chem. 1995 Sep 29;38(20):4044-55. doi: 10.1021/jm00020a020. J Med Chem. 1995. PMID: 7562940
-
Anticancer Potential of Coumarin and its Derivatives.Mini Rev Med Chem. 2021;21(19):2996-3029. doi: 10.2174/1389557521666210405160323. Mini Rev Med Chem. 2021. PMID: 33820507 Review.
-
In-vivo metabolism of 4-substituted arylpiperazines to pharmacologically active 1-arylpiperazines.Boll Chim Farm. 1990 May;129(5):183-9. Boll Chim Farm. 1990. PMID: 1982054 Review.
Cited by
-
Design, Synthesis, and Biological Evaluation of a Series of 5- and 7-Hydroxycoumarin Derivatives as 5-HT1A Serotonin Receptor Antagonists.Pharmaceuticals (Basel). 2021 Feb 24;14(3):179. doi: 10.3390/ph14030179. Pharmaceuticals (Basel). 2021. PMID: 33668396 Free PMC article.
-
New Piperazine Derivatives of 6-Acetyl-7-hydroxy-4-methylcoumarin as 5-HT1A Receptor Agents.Int J Mol Sci. 2023 Feb 1;24(3):2779. doi: 10.3390/ijms24032779. Int J Mol Sci. 2023. PMID: 36769117 Free PMC article.
-
Synthesis of Chromeno[3,4-b]piperazines by an Enol-Ugi/Reduction/Cyclization Sequence.Molecules. 2021 Feb 27;26(5):1287. doi: 10.3390/molecules26051287. Molecules. 2021. PMID: 33673443 Free PMC article.
-
Identification of a Novel Subtype-Selective α1B-Adrenoceptor Antagonist.ACS Chem Neurosci. 2024 Feb 7;15(3):671-684. doi: 10.1021/acschemneuro.3c00767. Epub 2024 Jan 18. ACS Chem Neurosci. 2024. PMID: 38238043 Free PMC article.
-
Design and synthesis of coumarin-triazole hybrids: biocompatible anti-diabetic agents, in silico molecular docking and ADME screening.Heliyon. 2020 Oct 17;6(10):e05290. doi: 10.1016/j.heliyon.2020.e05290. eCollection 2020 Oct. Heliyon. 2020. PMID: 33102875 Free PMC article.
References
-
- Abate A., Dimartino V., Spina P., Costa P.L., Lombardo C., Santini A., Del Piano M., Alimonti P. Hymecromone in the treatment of motor disorders of the bile ducts: a multicenter, double-blind, placebo-controlled clinical study. Drugs. Exp. Clin. Res. 2001;27:223–231. - PubMed
-
- Alvarez A., Bronfman F., Perez C.A., Vicente M., Garrido J., Inestrosa N.C. Acetylcholinesterase, a senile plaque component, affects the fibrillogenesis of amyloid-beta-peptides. Neurosci. Lett. 1995;201:49–52. - PubMed
-
- Anand P., Singh B., Singh N.A. Review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012;20:1175–1180. - PubMed
-
- Archier E., Devaux S., Castela E., Gallini A., Aubin F., Le Maître M., Aractingi S., Bachelez H., Cribier B., Joly P., Jullien D., Misery L., Paul C., Ortonne J.P., Richard M.A. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2012;26(Suppl 3):11–21. - PubMed
-
- Božič-Mijovski M. Advances in monitoring anticoagulant therapy. Adv. Clin. Chem. 2019;90:197–213. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

