Long-term efficacy and safety of subcutaneous C1-inhibitor in women with hereditary angioedema: subgroup analysis from an open-label extension of a phase 3 trial
- PMID: 32042283
- PMCID: PMC7001333
- DOI: 10.1186/s13223-020-0409-3
Long-term efficacy and safety of subcutaneous C1-inhibitor in women with hereditary angioedema: subgroup analysis from an open-label extension of a phase 3 trial
Abstract
Background: Women with hereditary angioedema due to C1-inhibitor deficiency (HAE-C1INH) experience more frequent and severe angioedema attacks compared with men. Fluctuations in female sex hormones can influence HAE attack frequency and severity. Subcutaneous C1-INH (C1-INH [SC]) is indicated as routine prophylaxis to prevent HAE attacks. In this post hoc subgroup analysis, we evaluated the efficacy and safety of C1-INH (SC) in female subjects with HAE-C1INH enrolled in an open-label extension of the pivotal phase III COMPACT trial.
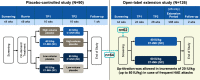
Methods: In this multicenter, randomized, parallel-arm trial, eligible subjects (age ≥ 6 years with ≥ 4 attacks over 2 consecutive months) received C1-INH (SC) 40 IU/kg or 60 IU/kg twice weekly for 52 to 140 weeks. Analyses of efficacy endpoints were performed for all female subjects and those of childbearing age (age ≥ 15 to ≤ 45 years), including subjects who became pregnant during the evaluation period.
Results: Overall, 91% (69/76) of female subjects were classified as responders (≥ 50% reduction in HAE attacks relative to the pre-study period); 82% experienced < 1 attack/4 weeks. The median number of attacks/month was 0.10, with 96% median reduction in attacks relative to the pre-study period. Results were similar in the subgroup of subjects of childbearing age. Four women who became pregnant during the trial and were exposed to C1-INH (SC) during the first trimester delivered healthy babies with no congenital abnormalities.
Conclusions: C1-INH (SC) prophylaxis was safe and effective in women with HAE-C1INH, including those of childbearing age. Four women exposed to C1-INH (SC) during the first trimester had uneventful pregnancies and delivered healthy babies.Trial registration Clinicaltrials.gov identifier NCT02316353 (Registered December 10, 2014); https://clinicaltrials.gov/ct2/show/NCT02316353.
Keywords: C1-inhibitor; Childbearing; Conception; Estrogen; Female; HAEGARDA; Hereditary angioedema; Pregnancy; Women.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsD. Levy has served on the speaker’s bureau, as a consultant, on a steering committee, and as a clinical investigator for CSL Behring; consultant for BioCryst; and speaker for Takeda. H. Farkas received institutional support for a clinical trial for this study from CSL Behring; advisory board/consultancy fees and/or speaker’s honoraria from BioCryst, CSL Behring, Shire, and Sobi (Swedish Orphan Biovitrum); and travel support from CSL Behring. M. Riedl reports grant support from CSL Behring during the conduct of the study and has received research grants from BioCryst, CSL Behring, Dyax, Ionis Pharmaceuticals, Pharming Technologies, and Shire; has served as a consultant and/or speaker for Adverum Biotechnologies, Alnylam Pharmaceuticals, Arrowhead Pharmaceuticals, BioCryst, CSL Behring, Dyax, Global Blood Therapeutics, Ionis Pharmaceuticals, KalVista Pharmaceuticals, Pharming Technologies, Salix Pharmaceuticals, and Shire; and is an uncompensated advisory board member for the US Hereditary Angioedema Association, outside the submitted work. F. Hsu reports serving as a consultant for BioCryst; serving as a speaker for CSL Behring, Pharming Technologies BV, and Takeda Pharmaceutical Company Ltd; and performing contracted research for Hoffman-La Roche. J.P. Brooks declares that he has no competing interests. M. Cicardi received grants from Shire and personal fees from Alnylam, BioCryst, CSL Behring, Dyax, KalVista, Pharming Technologies, Shire, Sobi (Swedish Orphan Biovitrum), and ViroPharma. H. Feuersenger and I. Pragst are employees of CSL Behring. A. Reshef reports grant support from CSL Behring during the conduct of the study and has received grant support from Pharming.
Figures
Similar articles
-
Subcutaneous C1 inhibitor for prevention of attacks of hereditary angioedema: additional outcomes and subgroup analysis of a placebo-controlled randomized study.Allergy Asthma Clin Immunol. 2019 Aug 28;15:49. doi: 10.1186/s13223-019-0362-1. eCollection 2019. Allergy Asthma Clin Immunol. 2019. PMID: 31485239 Free PMC article.
-
Long-term health-related quality of life in patients treated with subcutaneous C1-inhibitor replacement therapy for the prevention of hereditary angioedema attacks: findings from the COMPACT open-label extension study.Orphanet J Rare Dis. 2021 Feb 15;16(1):86. doi: 10.1186/s13023-020-01658-4. Orphanet J Rare Dis. 2021. PMID: 33588897 Free PMC article.
-
Long-Term Efficacy of Subcutaneous C1 Inhibitor in Pediatric Patients with Hereditary Angioedema.Pediatr Allergy Immunol Pulmonol. 2020 Sep 1;33(3):136-141. doi: 10.1089/ped.2020.1143. Epub 2020 Sep 16. Pediatr Allergy Immunol Pulmonol. 2020. PMID: 32953229 Free PMC article. Clinical Trial.
-
Preventive Treatment of Hereditary Angioedema: A Review of Phase III Clinical Trial Data for Subcutaneous C1 Inhibitor and Relevance for Patient Management.Clin Ther. 2021 Dec;43(12):2154-2166.e1. doi: 10.1016/j.clinthera.2021.10.008. Epub 2021 Dec 5. Clin Ther. 2021. PMID: 34879971 Review.
-
A Review of Randomized Controlled Trials of Hereditary Angioedema Long-Term Prophylaxis with C1 Inhibitor Replacement Therapy: Alleviation of Disease Symptoms Is Achievable.J Asthma Allergy. 2023 Mar 9;16:269-277. doi: 10.2147/JAA.S396338. eCollection 2023. J Asthma Allergy. 2023. PMID: 36922963 Free PMC article. Review.
Cited by
-
Plasma-derived C1 esterase inhibitor pharmacokinetics and safety in patients with hereditary angioedema.J Allergy Clin Immunol Glob. 2023 Nov 14;3(1):100178. doi: 10.1016/j.jacig.2023.100178. eCollection 2024 Feb. J Allergy Clin Immunol Glob. 2023. PMID: 38033485 Free PMC article.
-
Therapeutic management of hereditary angioedema: past, present, and future.Balkan Med J. 2021 Mar;38(2):89-103. doi: 10.5152/balkanmedj.2021.21094. Balkan Med J. 2021. PMID: 33724190 Free PMC article.
-
Reviewing clinical considerations and guideline recommendations of C1 inhibitor prophylaxis for hereditary angioedema.Clin Transl Allergy. 2022 Jan 18;12(1):e12092. doi: 10.1002/clt2.12092. eCollection 2022 Jan. Clin Transl Allergy. 2022. PMID: 35079346 Free PMC article. Review.
-
Subcutaneous C1-Inhibitor Concentrate for prophylaxis during pregnancy and lactation in a patient with C1-INH-HAE.Clin Case Rep. 2021 Feb 6;9(3):1273-1275. doi: 10.1002/ccr3.3743. eCollection 2021 Mar. Clin Case Rep. 2021. PMID: 33768824 Free PMC article.
-
Considerations in the management of hereditary angioedema due to C1-INH deficiency in women of childbearing age.Allergy Asthma Clin Immunol. 2022 Jul 13;18(1):64. doi: 10.1186/s13223-022-00689-9. Allergy Asthma Clin Immunol. 2022. PMID: 35831891 Free PMC article. Review.
References
-
- Agostoni A, Aygören-Pürsün E, Binkley KE, Blanch A, Bork K, Bouillet LC, et al. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004;114:S51–S131. doi: 10.1016/j.jaci.2004.06.047. - DOI - PMC - PubMed
-
- Bouillet L, Launay D, Fain O, Boccon-Gibod I, Laurent J, Martin L, et al. French National Reference Center for Hereditary Angioedema (CREAK). Hereditary angioedema with C1 inhibitor deficiency: clinical presentation and quality of life of 193 French patients. Ann Allergy Asthma Immunol. 2013;111:290–294. doi: 10.1016/j.anai.2013.07.012. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

