A randomized, double-blind, placebo-controlled, parallel-group study of once-daily inhaled fluticasone furoate on the hypothalamic-pituitary-adrenocortical axis of children with asthma
- PMID: 32042286
- PMCID: PMC7001316
- DOI: 10.1186/s13223-020-0406-6
A randomized, double-blind, placebo-controlled, parallel-group study of once-daily inhaled fluticasone furoate on the hypothalamic-pituitary-adrenocortical axis of children with asthma
Abstract
Background: To evaluate the effects of fluticasone furoate on the hypothalamic-pituitary-adrenocortical axis, and the safety and tolerability of fluticasone furoate treatment in children with asthma.
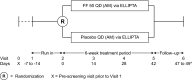
Methods: This was a randomized, double-blind, placebo-controlled, multicenter, stratified, parallel-group, non-inferiority study of fluticasone furoate 50 µg inhalation powder administered once daily. The study enrolled children (aged 5-11 years inclusive) with a documented diagnosis of asthma for ≥ 6 months and a Childhood Asthma Control Test score of > 19. After a 7-14-day run-in period, eligible subjects were stratified by age and randomized to fluticasone furoate 50 µg once daily or placebo once daily via ELLIPTA for 6 weeks. The primary endpoint was the change from baseline (expressed as a ratio) in 0-24-h weighted mean serum cortisol at the end of the treatment period.
Results: Fifty-six randomized subjects received fluticasone furoate 50 µg once daily and 55 received placebo. The primary analysis was performed in the serum cortisol population (n = 104) and demonstrated that fluticasone furoate 50 µg once daily was non-inferior to placebo (ratio = 0.93; 95% confidence interval 0.8096, 1.0620), as the lower limit of the 95% confidence interval for the geometric mean treatment ratio of fluticasone furoate 50 µg once daily versus placebo was greater than 0.80. Findings from the intent-to-treat population (n = 111) were similar.
Conclusions: Six weeks of treatment with inhaled fluticasone furoate 50 µg once daily had no clinically relevant effect on the hypothalamic-pituitary-adrenocortical axis function of children, as measured by 24-h serum cortisol profiles. The primary analysis showed that fluticasone furoate 50 µg once daily was non-inferior to placebo. Fluticasone furoate 50 µg once daily was well tolerated and no new safety concerns emerged during the study.
Trial registration: This study is registered in ClinicalTrials.gov (NCT02483975). Date of submission: 25 June 2015.
Keywords: Asthma; Fluticasone furoate (FF); Pediatric; Safety, HPA axis; Serum cortisol.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsPB, ST, VI, RM, and SK are employees of and hold shares in GSK. KD and MT have no competing interests to disclose.
Figures

Similar articles
-
Inhaled fluticasone furoate/vilanterol does not affect hypothalamic-pituitary-adrenal axis function in adolescent and adult asthma: randomised, double-blind, placebo-controlled study.Clin Respir J. 2013 Oct;7(4):397-406. doi: 10.1111/crj.12026. Epub 2013 Jun 5. Clin Respir J. 2013. PMID: 23578031 Free PMC article. Clinical Trial.
-
Comparative clinical pharmacology of mometasone furoate, fluticasone propionate and fluticasone furoate.Pulm Pharmacol Ther. 2022 Dec;77:102171. doi: 10.1016/j.pupt.2022.102171. Epub 2022 Oct 13. Pulm Pharmacol Ther. 2022. PMID: 36243386 Clinical Trial.
-
Randomized Trial of Once-Daily Fluticasone Furoate in Children with Inadequately Controlled Asthma.J Pediatr. 2016 Nov;178:246-253.e2. doi: 10.1016/j.jpeds.2016.08.010. Epub 2016 Sep 9. J Pediatr. 2016. PMID: 27622699 Clinical Trial.
-
Fluticasone furoate/vilanterol: a review of its use in patients with asthma.Drugs. 2015 Mar;75(4):407-18. doi: 10.1007/s40265-015-0354-5. Drugs. 2015. PMID: 25648266 Review.
-
Inhaled mometasone furoate: a review of its use in adults and adolescents with persistent asthma.Drugs. 2001;61(9):1325-50. doi: 10.2165/00003495-200161090-00011. Drugs. 2001. PMID: 11511026 Review.
References
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention. Updated 2018. https://ginasthma.org/wp-content/uploads/2019/01/2018-GINA.pdf. Accessed Jan 2020.
-
- GSK. ARNUITY_ELLIPTA Prescribing Information. 2018. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Presc.... Accessed Aug 2018.
Associated data
LinkOut - more resources
Full Text Sources
Medical

