A bioceramic scaffold composed of strontium-doped three-dimensional hydroxyapatite whiskers for enhanced bone regeneration in osteoporotic defects
- PMID: 32042323
- PMCID: PMC6993240
- DOI: 10.7150/thno.40103
A bioceramic scaffold composed of strontium-doped three-dimensional hydroxyapatite whiskers for enhanced bone regeneration in osteoporotic defects
Abstract
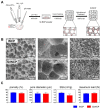
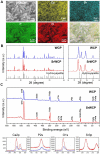
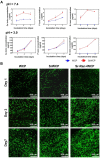
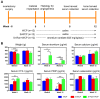
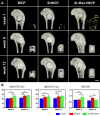
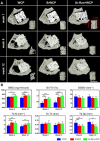
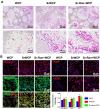
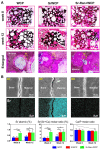
Reconstruction of osteoporotic bone defects is a clinical problem that continues to inspire the design of new materials. Methods: In this work, bioceramics composed of strontium (Sr)-doped hydroxyapatite (HA) whiskers or pure HA whiskers were successfully fabricated by hydrothermal treatment and respectively named SrWCP and WCP. Both bioceramics had similar three-dimensional (3D) porous structures and mechanical strengths, but the SrWCP bioceramic was capable of releasing Sr under physiological conditions. In an osteoporotic rat metaphyseal femoral bone defect model, both bioceramic scaffolds were implanted, and another group that received WCP plus strontium ranelate drug administration (Sr-Ran+WCP) was studied for comparison. Results: At week 1 post-implantation, osteogenesis coupled blood vessels were found to be more common in the SrWCP and Sr-Ran+WCP groups, with substantial vascular-like structures. After 12 weeks of implantation, comparable to the Sr-Ran+WCP group, the SrWCP group showed induction of more new bone formation within the defect as well as at the implant-bone gap region than that of the WCP group. Both the SrWCP and Sr-Ran+WCP groups yielded a beneficial effect on the surrounding trabecular bone microstructure to resist osteoporosis-induced progressive bone loss. While an abnormally high blood Sr ion concentration was found in the Sr-Ran+WCP group, SrWCP showed little adverse effect. Conclusion: Our results collectively suggest that the SrWCP bioceramic can be a safe bone substitute for the treatment of osteoporotic bone defects, as it promotes local bone regeneration and implant osseointegration to a level that strontium ranelate can achieve.
Keywords: bone regeneration; calcium phosphate bioceramics; osteoporosis; strontium; whiskerization..
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Intermittent administration of human parathyroid hormone (1-34) increases fixation of strontium-doped hydroxyapatite coating titanium implants via electrochemical deposition in ovariectomized rat femur.J Biomater Appl. 2016 Feb;30(7):952-60. doi: 10.1177/0885328215610898. Epub 2015 Oct 18. J Biomater Appl. 2016. PMID: 26482573
-
Strontium Hydroxyapatite scaffolds engineered with stem cells aid osteointegration and osteogenesis in osteoporotic sheep model.Colloids Surf B Biointerfaces. 2018 Mar 1;163:346-354. doi: 10.1016/j.colsurfb.2017.12.048. Epub 2017 Dec 28. Colloids Surf B Biointerfaces. 2018. PMID: 29331906
-
The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration.Acta Biomater. 2017 Oct 1;61:217-232. doi: 10.1016/j.actbio.2017.08.015. Epub 2017 Aug 12. Acta Biomater. 2017. PMID: 28807800
-
Strontium ranelate in osteoporosis.Curr Pharm Des. 2002;8(21):1907-16. doi: 10.2174/1381612023393639. Curr Pharm Des. 2002. PMID: 12171530 Review.
-
Strontium-doped hydroxyapatite and its role in osteogenesis and angiogenesis.Int J Dev Biol. 2023;67(4):137-146. doi: 10.1387/ijdb.230091lc. Int J Dev Biol. 2023. PMID: 37975329 Review.
Cited by
-
Engineering Multifunctional Hydrogel With Osteogenic Capacity for Critical-Size Segmental Bone Defect Repair.Front Bioeng Biotechnol. 2022 May 9;10:899457. doi: 10.3389/fbioe.2022.899457. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35615472 Free PMC article.
-
New strontium-based coatings show activity against pathogenic bacteria in spine infection.Front Bioeng Biotechnol. 2024 Apr 10;12:1347811. doi: 10.3389/fbioe.2024.1347811. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38665815 Free PMC article.
-
Strontium Peroxide-Loaded Composite Scaffolds Capable of Generating Oxygen and Modulating Behaviors of Osteoblasts and Osteoclasts.Int J Mol Sci. 2022 Jun 5;23(11):6322. doi: 10.3390/ijms23116322. Int J Mol Sci. 2022. PMID: 35683001 Free PMC article.
-
Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis.Bioact Mater. 2021 Sep 16;10:195-206. doi: 10.1016/j.bioactmat.2021.09.013. eCollection 2022 Apr. Bioact Mater. 2021. PMID: 34901539 Free PMC article.
-
In situ construction of flower-like nanostructured calcium silicate bioceramics for enhancing bone regeneration mediated via FAK/p38 signaling pathway.J Nanobiotechnology. 2022 Mar 27;20(1):162. doi: 10.1186/s12951-022-01361-5. J Nanobiotechnology. 2022. PMID: 35351145 Free PMC article.
References
-
- Muruganandan S, Dranse HJ, Rourke JL, McMullen NM, Sinal CJ. Chemerin neutralization blocks hematopoietic stem cell osteoclastogenesis. Stem Cells. 2013;31:2172–82. - PubMed
-
- Hu Y, Li J, Zhu X, Li Y, Zhang S, Chen X. et al. 17β-Estradiol-Loaded PEGlyated Upconversion Nanoparticles as a Bone-Targeted Drug Nanocarrier. ACS Appl Mater Interfaces. 2015;7:15803–11. - PubMed
-
- García-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

