Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats
- PMID: 32042324
- PMCID: PMC6993237
- DOI: 10.7150/thno.36272
Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats
Abstract
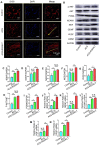
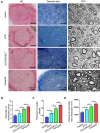
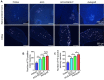
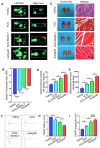
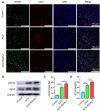
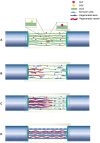
Autologous nerve transplantation, which is the gold standard for clinical treatment of peripheral nerve injury, still has many limitations. In this study, aligned chitosan fiber hydrogel (ACG) grafted with a bioactive peptide mixture consisting of RGI (Ac-RGIDKRHWNSQGG) and KLT (Ac-KLTWQELYQLKYKGIGG), designated as ACG-RGI/KLT, was used as nerve conduit filler to repair sciatic nerve defects in rats. Methods: Chitosan nanofiber hydrogel was prepared by a combination of electrospinning and mechanical stretching methods, and was then grafted with RGI and KLT, which are peptides mimicking brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), respectively. The physicochemical properties of ACG-RGI/KLT were fully characterized. In vitro, the distribution, proliferation, and secretory activity of Schwann cells were analyzed. Next, the in vivo repair potential for 15-mm rat sciatic nerve defects was examined. The recovery of regenerated nerve, muscle, and motor function was evaluated by neuromuscular histology, electrophysiology, and catwalk gait analysis. Results: We first constructed directionally aligned chitosan nanofiber hydrogel grafted with RGI/KLT peptide mixture (ACG-RGI/KLT). ACG-RGI/KLT oriented the Schwann cells, and promoted the proliferation and secretion of neurotrophic factors by Schwann cells. At an early injury stage, ACG-RGI/KLT not only enhanced nerve regeneration, but also promoted vascular penetration. At 12 weeks, ACG-RGI/KLT facilitated nerve regeneration and functional recovery in rats. Conclusions: Aligned chitosan nanofiber hydrogel grafted with RGI/KLT peptide provides an effective means of repairing sciatic nerve defects and shows great potential for clinical application.
Keywords: Peripheral nerve injury; chitosan nanofibers; electrospinning; mechanical stretching; peptides..
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Self-assembling peptide hydrogels functionalized with LN- and BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration.Theranostics. 2020 Jul 9;10(18):8227-8249. doi: 10.7150/thno.44276. eCollection 2020. Theranostics. 2020. PMID: 32724468 Free PMC article.
-
Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel.Acta Biomater. 2017 Jun;55:296-309. doi: 10.1016/j.actbio.2017.04.010. Epub 2017 Apr 12. Acta Biomater. 2017. PMID: 28412554
-
Effects of Schwann cell alignment along the oriented electrospun chitosan nanofibers on nerve regeneration.J Biomed Mater Res A. 2009 Dec 15;91(4):994-1005. doi: 10.1002/jbm.a.32329. J Biomed Mater Res A. 2009. PMID: 19097155
-
Harnessing the Potential of Self-Assembled Peptide Hydrogels for Neural Regeneration and Tissue Engineering.Macromol Biosci. 2024 Jun;24(6):e2300534. doi: 10.1002/mabi.202300534. Epub 2024 Apr 9. Macromol Biosci. 2024. PMID: 38547473 Review.
-
Chitosan conduits for peripheral nerve repair: a systematic review of animal studies.Braz J Biol. 2025 Apr 4;84:e280569. doi: 10.1590/1519-6984.280569. eCollection 2025. Braz J Biol. 2025. PMID: 40197895
Cited by
-
A hyaluronic acid granular hydrogel nerve guidance conduit promotes regeneration and functional recovery of injured sciatic nerves in rats.Neural Regen Res. 2023 Mar;18(3):657-663. doi: 10.4103/1673-5374.350212. Neural Regen Res. 2023. PMID: 36018191 Free PMC article.
-
Biophysical and Biochemical Cues of Biomaterials Guide Mesenchymal Stem Cell Behaviors.Front Cell Dev Biol. 2021 Mar 25;9:640388. doi: 10.3389/fcell.2021.640388. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33842464 Free PMC article. Review.
-
Artificial nerve graft constructed by coculture of activated Schwann cells and human hair keratin for repair of peripheral nerve defects.Neural Regen Res. 2023 May;18(5):1118-1123. doi: 10.4103/1673-5374.355817. Neural Regen Res. 2023. PMID: 36255001 Free PMC article.
-
Engineering multifunctional bioactive citrate-based biomaterials for tissue engineering.Bioact Mater. 2022 May 7;19:511-537. doi: 10.1016/j.bioactmat.2022.04.027. eCollection 2023 Jan. Bioact Mater. 2022. PMID: 35600971 Free PMC article. Review.
-
Micron track chitosan conduit fabricated by 3D-printed model topography provides bionic microenvironment for peripheral nerve regeneration.Int J Bioprint. 2023 Jun 12;9(5):770. doi: 10.18063/ijb.770. eCollection 2023. Int J Bioprint. 2023. PMID: 37608847 Free PMC article.
References
-
- Asplund M, Nilsson M, Jacobsson A, von Holst H. Incidence of traumatic peripheral nerve injuries and amputations in Sweden between 1998 and 2006. Neuroepidemiology. 2009;32:217–28. - PubMed
-
- Bell JH, Haycock JW. Next generation nerve guides: materials, fabrication, growth factors, and cell delivery. Tissue Eng Part B Rev. 2012;18:116–28. - PubMed
-
- Chen WM, Chen S, Morsi Y, El-Hamshary H, El-Newehy M, Fan CY. et al. Superabsorbent 3D Scaffold Based on Electrospun Nanofibers for Cartilage Tissue Engineering. Acs Appl Mater Inter. 2016;8:24415–25. - PubMed
-
- Chiono V, Tonda-Turo C, Ciardelli G. Chapter 9: Artificial scaffolds for peripheral nerve reconstruction. Int Rev Neurobiol. 2009;87:173–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

