Sex Differences Revealed in a Mouse CFA Inflammation Model with Macrophage Targeted Nanotheranostics
- PMID: 32042330
- PMCID: PMC6993234
- DOI: 10.7150/thno.41309
Sex Differences Revealed in a Mouse CFA Inflammation Model with Macrophage Targeted Nanotheranostics
Abstract
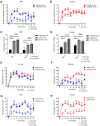
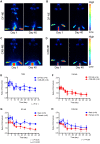
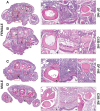
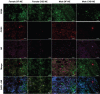
Monocyte derived macrophages (MDMs) infiltrate sites of infection or injury and upregulate cyclooxygenase-2 (COX-2), an enzyme that stimulates prostaglandin-E2 (PgE2). Nanotheranostics combine therapeutic and diagnostic agents into a single nanosystem. In previous studies, we demonstrated that a nanotheranostic strategy, based on theranostic nanoemulsions (NE) loaded with a COX-2 inhibitor (celecoxib, CXB) and equipped with near-infrared fluorescent (NIRF) reporters, can specifically target circulating monocytes and MDMs. The anti-inflammatory and anti-nociceptive effects of such cell-specific COX-2 inhibition lasted several days following Complete Freund's Adjuvant (CFA) or nerve injury in male mice. The overall goal of this study was to investigate the extended (up to 40 days) impact of MDM-targeted COX-2 inhibition and any sex-based differences in treatment response; both of which remain unknown. Our study also evaluates the feasibility and efficacy of a preclinical nanotheranostic strategy for mechanistic investigation of the impact of such sex differences on clinical outcomes. Methods: CFA was administered into the right hind paws of male and female mice. All mice received a single intravenous dose of NIRF labeled CXB loaded NE twelve hours prior to CFA injection. In vivo whole body NIRF imaging and mechanical hypersensitivity assays were performed sequentially and ex vivo NIRF imaging and immunohistopathology of foot pad tissues were performed at the end point of 40 days. Results: Targeted COX-2 inhibition of MDMs in male and female mice successfully improved mechanical hypersensitivity after CFA injury. However, we observed distinct sex-specific differences in the intensity or longevity of the nociceptive responses. In males, a single dose of CXB-NE administered via tail vein injection produced significant improved mechanical hypersensitivity for 32 days as compared to the drug free NE (DF-NE) (untreated) control group. In females, CXB-NE produced similar, though less prominent and shorter-lived effects, lasting up to 11 days. NIRF imaging confirmed that CXB-NE can be detected up to day 40 in the CFA injected foot pad tissues of both sexes. There were distinct signal distribution trends between males and females, suggesting differences in macrophage infiltration dynamics between the sexes. This may also relate to differences in macrophage turnover rate between the sexes, a possibility that requires further investigation in this model. Conclusions: For the first time, this study provides unique insight into MDM dynamics and the early as well as longer-term targeted effects and efficacy of a clinically translatable nanotheranostic agent on MDM mediated inflammation. Our data supports the potential of nanotheranostics as presented in elucidating the kinetics, dynamics and sex-based differences in the adaptive or innate immune responses to inflammatory triggers. Taken together, our study findings lead us closer to true personalized, sex-specific pain nanomedicine for a wide range of inflammatory diseases.
Keywords: Pain nanomedicine; inflammatory pain; macrophages; nanotheranostic.; sex differences.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Complete Freund's adjuvant-induced reduction of exploratory activity in a novel environment as an objective nociceptive endpoint for sub-acute inflammatory pain model in rats.Eur J Pain. 2015 Nov;19(10):1527-36. doi: 10.1002/ejp.686. Epub 2015 Mar 2. Eur J Pain. 2015. PMID: 25731687
-
Theranostic nanoemulsions for macrophage COX-2 inhibition in a murine inflammation model.Clin Immunol. 2015 Sep;160(1):59-70. doi: 10.1016/j.clim.2015.04.019. Epub 2015 May 8. Clin Immunol. 2015. PMID: 25959685 Free PMC article.
-
Nanomedicine-driven neuropathic pain relief in a rat model is associated with macrophage polarity and mast cell activation.Acta Neuropathol Commun. 2019 Jul 5;7(1):108. doi: 10.1186/s40478-019-0762-y. Acta Neuropathol Commun. 2019. PMID: 31277709 Free PMC article.
-
Cutaneous Application of Celecoxib for Inflammatory and Cancer Diseases.Curr Cancer Drug Targets. 2019;19(1):5-16. doi: 10.2174/1568009618666180430125201. Curr Cancer Drug Targets. 2019. PMID: 29714143 Review.
-
Sex-differences in prostaglandin signaling: a semi-systematic review and characterization of PTGDS expression in human sensory neurons.Sci Rep. 2023 Mar 22;13(1):4670. doi: 10.1038/s41598-023-31603-x. Sci Rep. 2023. PMID: 36949072 Free PMC article. Review.
Cited by
-
In vitro Quality Assessments of Perfluorocarbon Nanoemulsions for Near-infrared Fluorescence Imaging of Inflammation in Preclinical Models.Bio Protoc. 2023 Oct 5;13(19):e4842. doi: 10.21769/BioProtoc.4842. eCollection 2023 Oct 5. Bio Protoc. 2023. PMID: 37817906 Free PMC article.
-
Sex Differences in CGRP Regulation and Function in the Amygdala in a Rat Model of Neuropathic Pain.Front Mol Neurosci. 2022 Jun 3;15:928587. doi: 10.3389/fnmol.2022.928587. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35726298 Free PMC article.
-
RNA-Seq Reveals Sex Differences in Gene Expression during Peripheral Neuropathic Inflammation and in Pain Relief from a COX-2 Inhibiting Theranostic Nanoemulsion.Int J Mol Sci. 2023 May 23;24(11):9163. doi: 10.3390/ijms24119163. Int J Mol Sci. 2023. PMID: 37298117 Free PMC article.
-
Theranostic nanoemulsions suppress macrophage-mediated acute inflammation in rats.J Nanobiotechnology. 2025 Feb 4;23(1):80. doi: 10.1186/s12951-025-03164-w. J Nanobiotechnology. 2025. PMID: 39905487 Free PMC article.
-
Low-Frequency Stimulation of Trpv1-Lineage Peripheral Afferents Potentiates the Excitability of Spino-Periaqueductal Gray Projection Neurons.J Neurosci. 2024 Jan 17;44(3):e1184232023. doi: 10.1523/JNEUROSCI.1184-23.2023. J Neurosci. 2024. PMID: 38050062 Free PMC article.
References
-
- Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nature reviews Cancer. 2002;2:201–9. - PubMed
-
- Davignon JL, Hayder M, Baron M, Boyer JF, Constantin A, Apparailly F. et al. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2013;52:590–8. - PubMed
-
- Siouti E, Andreakos E. The many facets of macrophages in rheumatoid arthritis. Biochem Pharmacol; 2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

