UCH-L1-mediated Down-regulation of Estrogen Receptor α Contributes to Insensitivity to Endocrine Therapy for Breast Cancer
- PMID: 32042339
- PMCID: PMC6993235
- DOI: 10.7150/thno.39814
UCH-L1-mediated Down-regulation of Estrogen Receptor α Contributes to Insensitivity to Endocrine Therapy for Breast Cancer
Abstract
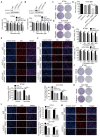
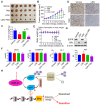
Purpose: To determine the role of UCH-L1 in regulating ERα expression, and to evaluate whether therapeutic targeting of UCH-L1 can enhance the efficacy of anti-estrogen therapy against breast cancer with loss or reduction of ERα. Methods: Expressions of UCH-L1 and ERα were examined in breast cancer cells and patient specimens. The associations between UCH-L1 and ERα, therapeutic response and prognosis in breast cancer patients were analyzed using multiple databases. The molecular pathways by which UCH-L1 regulates ERα were analyzed using immunoblotting, qRT-PCR, immunoprecipitation, ubiquitination, luciferase and ChIP assays. The effects of UCH-L1 inhibition on the efficacy of tamoxifen in ERα (-) breast cancer cells were tested both in vivo and in vitro. Results: UCH-L1 expression was conversely correlated with ERα status in breast cancer, and the negative regulatory effect of UCH-L1 on ERα was mediated by the deubiquitinase-mediated stability of EGFR, which suppresses ERα transcription. High expression of UCH-L1 was associated with poor therapeutic response and prognosis in patients with breast cancer. Up-regulation of ERα caused by UCH-L1 inhibition could significantly enhance the efficacy of tamoxifen and fulvestrant in ERα (-) breast cancer both in vivo and in vitro. Conclusions: Our results reveal an important role of UCH-L1 in modulating ERα status and demonstrate the involvement of UCH-L1-EGFR signaling pathway, suggesting that UCH-L1 may serve as a novel adjuvant target for treatment of hormone therapy-insensitive breast cancers. Targeting UCH-L1 to sensitize ER negative breast cancer to anti-estrogen therapy might represent a new therapeutic strategy that warrants further exploration.
Keywords: EGFR; ER-negative breast cancer; Endocrine therapy; Estrogen receptor α; Ubiquitin carboxyl terminal hydrolase-L1.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
USP36 promotes tumorigenesis and tamoxifen resistance in breast cancer by deubiquitinating and stabilizing ERα.J Exp Clin Cancer Res. 2024 Aug 31;43(1):249. doi: 10.1186/s13046-024-03160-2. J Exp Clin Cancer Res. 2024. PMID: 39215346 Free PMC article.
-
UCH-L1 involved in regulating the degradation of EGFR and promoting malignant properties in drug-resistant breast cancer.Int J Clin Exp Pathol. 2015 Oct 1;8(10):12500-8. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26722437 Free PMC article.
-
Menin, a product of the MENI gene, binds to estrogen receptor to enhance its activity in breast cancer cells: possibility of a novel predictive factor for tamoxifen resistance.Breast Cancer Res Treat. 2010 Jul;122(2):395-407. doi: 10.1007/s10549-009-0581-0. Epub 2009 Oct 22. Breast Cancer Res Treat. 2010. PMID: 19847644
-
Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer.Endocr Relat Cancer. 2005 Dec;12(4):721-47. doi: 10.1677/erc.1.00857. Endocr Relat Cancer. 2005. PMID: 16322319 Review.
-
The use of selective estrogen receptor modulators and selective estrogen receptor down-regulators in breast cancer.Best Pract Res Clin Endocrinol Metab. 2004 Mar;18(1):47-66. doi: 10.1016/j.beem.2003.08.002. Best Pract Res Clin Endocrinol Metab. 2004. PMID: 14687597 Review.
Cited by
-
Stabilization of estrogen receptor α by USP37 contributes to the progression of breast cancer.Cancer Sci. 2023 May;114(5):2041-2052. doi: 10.1111/cas.15613. Epub 2023 Mar 10. Cancer Sci. 2023. PMID: 36221793 Free PMC article.
-
Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor A (VEGF-A) expressions in Ethiopian female breast cancer and their association with histopathologic features.PLoS One. 2024 Oct 15;19(10):e0308411. doi: 10.1371/journal.pone.0308411. eCollection 2024. PLoS One. 2024. PMID: 39405290 Free PMC article.
-
Progress of Breast Cancer basic research in China.Int J Biol Sci. 2021 May 11;17(8):2069-2079. doi: 10.7150/ijbs.60631. eCollection 2021. Int J Biol Sci. 2021. PMID: 34131406 Free PMC article. Review.
-
Recent advances in anti-inflammatory active components and action mechanisms of natural medicines.Inflammopharmacology. 2023 Dec;31(6):2901-2937. doi: 10.1007/s10787-023-01369-9. Epub 2023 Nov 10. Inflammopharmacology. 2023. PMID: 37947913 Review.
-
The deubiquitinating enzyme USP15 stabilizes ERα and promotes breast cancer progression.Cell Death Dis. 2021 Mar 26;12(4):329. doi: 10.1038/s41419-021-03607-w. Cell Death Dis. 2021. PMID: 33771975 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin; 2018. - PubMed
-
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. - PubMed
-
- Dahlman-Wright K, Cavailles V, Fuqua SA. et al. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–81. - PubMed
-
- Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

