Cancer/testis antigens expression during cultivation of melanoma and soft tissue sarcoma cells
- PMID: 32042403
- PMCID: PMC6998350
- DOI: 10.1186/s13569-020-0125-2
Cancer/testis antigens expression during cultivation of melanoma and soft tissue sarcoma cells
Abstract
Background: Autologous dendritic cells (DC) loaded with tumor-associated antigens (TAAs) are a promising approach for anticancer immunotherapy. Polyantigen lysates appear to be an excellent source of TAAs for loading onto the patient's dendritic cells. Cancer/testis antigens (CTA) are expressed by a wide range of tumors, but are minimally expressed on normal tissues, and could serve as a universal target for immunotherapy. However, CTA expression levels can vary significantly in patients with the same tumor type. We proposed that patients who do not respond to DC-based therapy may have distinct features of the CTA expression profile on tumor cells.
Patients and methods: We compared the gene expression of the principal families CTA in 22 melanoma and 27 soft tissue and bone sarcomas cell lines (STBS), received from patients and used for DC vaccine preparation.
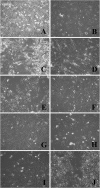
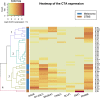
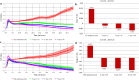
Results: The majority (47 of 49, 95.9%) cell lines showed CTA gene activity. The incidence of gene expression of GAGE, NYESO1, MAGEA1, PRAME's was significantly different (adj. p < 0.05) between melanoma and sarcoma cell lines. The expression of the SCP1 gene was detected neither in melanoma cells nor in the STBS cells. Clustering by the gene expression profile revealed four different expression patterns. We found three main patterns types: hyperexpression of multiple CTA, hyperexpression of one CTA with almost no expression of others, and no expression of CTA. All clusters types exist in melanoma and sarcoma cell lines. We observed dependence of killing efficacy from the PRAME (rho = 0.940, adj. p < 0.01) expression during real-time monitoring with the xCELLigence system of the interaction between melanoma or sarcoma cells with the T-lymphocytes activated by the lysate of selected allogenous melanoma cell lines with high expression of CTA.
Conclusion: Our results demonstrate that one can use lysates from allogeneic melanoma cell lines as a source of CTA for DC load during the production of anticancer vaccines for the STBS treatment. Patterns of CTA expression should be evaluated as biomarkers of response in prospective clinical trials.
Keywords: Cancer/testis antigens; Dendritic cell vaccine; Melanoma; Soft and bone tissues sarcoma; Tumor cells lines.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


Similar articles
-
Cancer/Testis Antigens: Expression, Regulation, Tumor Invasion, and Use in Immunotherapy of Cancers.Immunol Invest. 2016 Oct;45(7):619-40. doi: 10.1080/08820139.2016.1197241. Epub 2016 Sep 7. Immunol Invest. 2016. PMID: 27603913 Review.
-
Cancer-testis antigen expression in synovial sarcoma: NY-ESO-1, PRAME, MAGEA4, and MAGEA1.Hum Pathol. 2017 Mar;61:130-139. doi: 10.1016/j.humpath.2016.12.006. Epub 2016 Dec 16. Hum Pathol. 2017. PMID: 27993576
-
Biological features of tissue and bone sarcomas investigated using an in vitro model of clonal selection.Pathol Res Pract. 2021 Jan;217:153214. doi: 10.1016/j.prp.2020.153214. Epub 2020 Sep 13. Pathol Res Pract. 2021. PMID: 33290900
-
Tumor cell lysates as immunogenic sources for cancer vaccine design.Hum Vaccin Immunother. 2014;10(11):3261-9. doi: 10.4161/21645515.2014.982996. Hum Vaccin Immunother. 2014. PMID: 25625929 Free PMC article. Review.
-
NYESO-1/LAGE-1s and PRAME are targets for antigen specific T cells in chondrosarcoma following treatment with 5-Aza-2-deoxycitabine.PLoS One. 2012;7(2):e32165. doi: 10.1371/journal.pone.0032165. Epub 2012 Feb 27. PLoS One. 2012. PMID: 22384167 Free PMC article.
Cited by
-
Proteogenomic Analysis Unveils the HLA Class I-Presented Immunopeptidome in Melanoma and EGFR-Mutant Lung Adenocarcinoma.Mol Cell Proteomics. 2021;20:100136. doi: 10.1016/j.mcpro.2021.100136. Epub 2021 Aug 13. Mol Cell Proteomics. 2021. PMID: 34391887 Free PMC article.
-
Interrogating Epigenome toward Personalized Approach in Cutaneous Melanoma.J Pers Med. 2021 Sep 9;11(9):901. doi: 10.3390/jpm11090901. J Pers Med. 2021. PMID: 34575678 Free PMC article. Review.
-
Implementation of Vaccinomics and In-Silico Approaches to Construct Multimeric Based Vaccine Against Ovarian Cancer.Int J Pept Res Ther. 2021;27(4):2845-2859. doi: 10.1007/s10989-021-10294-w. Epub 2021 Oct 19. Int J Pept Res Ther. 2021. PMID: 34690620 Free PMC article.
References
LinkOut - more resources
Full Text Sources

