Development and proof-of-concept of a multicenter, patient-centered cancer registry for breast cancer patients with metastatic disease-the "Breast cancer care for patients with metastatic disease" (BRE-4-MED) registry
- PMID: 32042437
- PMCID: PMC7001276
- DOI: 10.1186/s40814-019-0541-3
Development and proof-of-concept of a multicenter, patient-centered cancer registry for breast cancer patients with metastatic disease-the "Breast cancer care for patients with metastatic disease" (BRE-4-MED) registry
Abstract
Background: Patients with metastatic breast cancer (MBC) are treated with a palliative approach with focus on controlling for disease symptoms and maintaining high quality of life. Information on individual needs of patients and their relatives as well as on treatment patterns in clinical routine care for this specific patient group are lacking or are not routinely documented in established Cancer Registries. Thus, we developed a registry concept specifically adapted for these incurable patients comprising primary and secondary data as well as mobile-health (m-health) data.
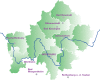
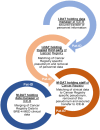
Methods: The concept for patient-centered "Breast cancer care for patients with metastatic disease" (BRE-4-MED) registry was developed and piloted exemplarily in the region of Main-Franconia, a mainly rural region in Germany comprising about 1.3 M inhabitants. The registry concept includes data on diagnosis, therapy, progression, patient-reported outcome measures (PROMs), and needs of family members from several sources of information including routine data from established Cancer Registries in different federal states, treating physicians in hospital as well as in outpatient settings, patients with metastatic breast cancer and their family members. Linkage with routine cancer registry data was performed to collect secondary data on diagnosis, therapy, and progression. Paper and online-based questionnaires were used to assess PROMs. A dedicated mobile application software (APP) was developed to monitor needs, progression, and therapy change of individual patients. Patient's acceptance and feasibility of data collection in clinical routine was assessed within a proof-of-concept study.
Results: The concept for the BRE-4-MED registry was developed and piloted between September 2017 and May 2018. In total n = 31 patients were included in the pilot study, n = 22 patients were followed up after 1 month. Record linkage with the Cancer Registries of Bavaria and Baden-Württemberg demonstrated to be feasible. The voluntary APP/online questionnaire was used by n = 7 participants. The feasibility of the registry concept in clinical routine was positively evaluated by the participating hospitals.
Conclusion: The concept of the BRE-4-MED registry provides evidence that combinatorial evaluation of PROMs, needs of family members, and raising clinical parameters from primary and secondary data sources as well as m-health applications are feasible and accepted in an incurable cancer collective.
Keywords: Health care service research; Metastatic breast cancer; Patient-centered registry; Patient’s needs; m-Health.
© The Author(s). 2020.
Conflict of interest statement
Competing interestsStephanie Stangl reports no disclosures. Kirsten Haas reports no disclosures. Felizitas Eichner reports no disclosures. Anna Grau reports no disclosures. Udo Selig reports no disclosures. Timo Ludwig reports no disclosures. Tanja Fehm reports no disclosures. Tanja Stüber reports no disclosure Asarnusch Rashid reports no disclosures. Alexander Kerscher reports no disclosures. Ralf Bargou reports no disclosures. Silke Hermann reports no disclosures. Volker Arndt reports no disclosures. Martin Meyer reports no disclosures. Manfred Wildner reports no disclosures. Hermann Faller reports no disclosures. Michael G. Schrauder reports no disclosures. Michael Weigel reports no disclosures. Ulrich Schlembach reports no disclosures. Peter U. Heuschmann reports research grants from German Ministry of Research and Education, German Research Foundation, European Union, Charité–Universitätsmedizin Berlin, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert Koch Institute, German Heart Foundation, University Göttingen (within FIND-AF randomized, supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), University Hospital Heidelberg (within RASUNOA-prime, supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo), grants from Charité–Universitätsmedizin Berlin (within Mondafis, supported by an unrestricted research grant to the Charité from Bayer), outside the submitted work. Achim Wöckel reports grant from German Ministry of Research and Education, received honoraria for consultancy and presentation from Amgen, Novartis, Eisai, Celgene, Pfizer, Tesaro, Aurikamed, TEVA, Lilly, and Roche within the last 5 years.
Figures


References
-
- Jamtvedt G, Young JM, Kristoffersen DT, et al. Audit and feedback: effects on professional practice and health care outcomes. The Cochrane database of systematic reviews 2006(2):Cd000259. 10.1002/14651858.CD000259. pub2[published Online First: Epub Date]|. - PubMed
-
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF). S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms. Version 4.1. 2017. AWMF Registernummer: 032-045OL, 2017.
-
- (NICE) NIfHaCE. Early and locally advanced breast cancer: diagnosis and management. Secondary EARLY and locally advanced breast cancer: diagnosis and management 2018. https://www.nice.org.uk/guidance/ng101.
LinkOut - more resources
Full Text Sources

