Very low dose naltrexone in opioid detoxification: a double-blind, randomized clinical trial of efficacy and safety
- PMID: 32042711
- PMCID: PMC6990297
- DOI: 10.1007/s43188-019-00008-2
Very low dose naltrexone in opioid detoxification: a double-blind, randomized clinical trial of efficacy and safety
Abstract
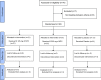
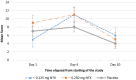
Withdrawal syndrome is one of the initial focuses of opioid detoxification. Very low dose naltrexone (VLNTX) has been found to reduce opioid tolerance and dependence in animal and human clinical studies. The aim of this study was to determine the safety and efficacy of VLNTX during early stages of detoxification. In a multi-arm parallel, double-blind, randomized controlled trial, 63 opioid-dependent male participants referring to Imam Reza Rehabilitation Center were allocated to three equal groups using block randomization method. They received 0.125 mg, 0.250 mg of VLNTX or placebo daily for 10 days, together with the routine clonidine-based protocol. Self-reported and observer ratings of withdrawal severity and adverse events were measured on the 1st, 4th and 10th day of treatment. Runny eyes (p = 0.006), anxiety (p = 0.031) and dehydration (p = 0.014) were reduced during the whole 10 days in the 0.125 mg VLNTX-treated group compared to placebo. Only drowsiness (p = 0.043) and dysphoric mood (p < 0.001) were reduced in the 0.250 mg VLNTX-treated group. Results of 1st, 4th, and 10th-day assessment showed that most symptoms reductions were for the 0.125 mg VLNTX and the placebo group in the 1st and 4th days, respectively. On the 10th day, there was not any significant difference between 0.250 mg VLNTX-treated group and placebo group. No adverse effect was observed. In the starting days of detoxification, VLNTX can reduce the withdrawal symptoms, but the efficacy declined by passing time. Further studies are needed to test the utility of this new therapeutic approach.
Keywords: Metabolic detoxification; Naltrexone; Opioid; Substance withdrawal syndrome.
© Korean Society of Toxicology 2019.
Conflict of interest statement
Conflict of interestAuthors have no conflict of interest to declare.
Figures
Similar articles
-
The combination very low-dose naltrexone-clonidine in the management of opioid withdrawal.Am J Drug Alcohol Abuse. 2012 May;38(3):200-5. doi: 10.3109/00952990.2011.644003. Epub 2012 Jan 10. Am J Drug Alcohol Abuse. 2012. PMID: 22233189 Free PMC article. Clinical Trial.
-
Problem drinking and low-dose naltrexone-assisted opioid detoxification.J Stud Alcohol Drugs. 2011 May;72(3):507-13. doi: 10.15288/jsad.2011.72.507. J Stud Alcohol Drugs. 2011. PMID: 21513688 Free PMC article. Clinical Trial.
-
Very low dose naltrexone addition in opioid detoxification: a randomized, controlled trial.Addict Biol. 2009 Apr;14(2):204-13. doi: 10.1111/j.1369-1600.2008.00119.x. Epub 2008 Aug 19. Addict Biol. 2009. PMID: 18715283 Free PMC article. Clinical Trial.
-
Early outcomes following low dose naltrexone enhancement of opioid detoxification.Am J Addict. 2009 Mar-Apr;18(2):109-16. doi: 10.1080/10550490902772785. Am J Addict. 2009. PMID: 19283561 Free PMC article. Clinical Trial.
-
Lofexidine, an {alpha}2-receptor agonist for opioid detoxification.Ann Pharmacother. 2010 Feb;44(2):343-51. doi: 10.1345/aph.1M347. Epub 2009 Dec 29. Ann Pharmacother. 2010. PMID: 20040696 Review.
Cited by
-
Use of Contrave, Naltrexone with Bupropion, Bupropion, or Naltrexone and Major Adverse Cardiovascular Events: A Systematic Literature Review.Diabetes Metab Syndr Obes. 2022 Sep 29;15:3049-3067. doi: 10.2147/DMSO.S381652. eCollection 2022. Diabetes Metab Syndr Obes. 2022. PMID: 36200062 Free PMC article. Review.
-
Opiate Antagonists for Chronic Pain: A Review on the Benefits of Low-Dose Naltrexone in Arthritis versus Non-Arthritic Diseases.Biomedicines. 2023 Jun 2;11(6):1620. doi: 10.3390/biomedicines11061620. Biomedicines. 2023. PMID: 37371715 Free PMC article. Review.
References
-
- Afshari R, Shafaeeyan H, Afshari R. An epidemiologic study of opioid dependent subjects who were volunteered for opioid detoxification in Iran, 2005. Clin Toxicol. 2006;44:581.
-
- Iran Drug Control Headquarters (2014) Statistics of addiction in Iran. http://www.dchq.ir. Accessed Sept 2014
LinkOut - more resources
Full Text Sources
Other Literature Sources