Cholangiocarcinoma: anatomical location-dependent clinical, prognostic, and genetic disparities
- PMID: 32042760
- PMCID: PMC6989985
- DOI: 10.21037/atm.2019.12.37
Cholangiocarcinoma: anatomical location-dependent clinical, prognostic, and genetic disparities
Abstract
Background: Anatomical location is considered in diagnostic and therapeutic approaches of cholangiocarcinoma (CCA). However, disparities and its extents in proportion of surgical candidates, prognostic factors, prognostic genetic networks, susceptibility for lymph node dissection, and disease stage at diagnosis remain to be confirmed.
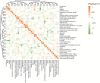
Methods: A total of 11,710 patients with cholangiocarcinoma from Surveillance, Epidemiology, and End Results Cancer Registries (SEER) and 45 CCA patients with paired tumor and normal specimens from The Cancer Genome Atlas were studied. Kaplan-Meier estimation, Cox proportional hazards regression, Pearson's correlation, comparison between anatomical location (distal, intrahepatic, and perihilar)-dependent CCAs, differential expressive gene stratification, potential interactive gene identification, and confirmation on pathways of the prognostic networks were carried out.
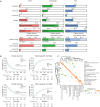
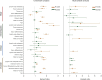
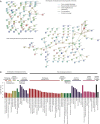
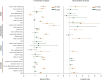
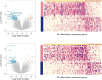
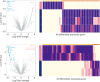
Results: Survival outcomes were most favorable in the distal type, followed by perihilar and intrahepatic types, but postsurgical prognosis was slightly higher in intrahepatic type compared to perihilar type. Distant historic stage at diagnosis was noticed in intrahepatic type. Significant prognostic factors and their hazards ratios were dependent to the anatomical location. In addition, lymph node dissection provided significant survival benefits in perihilar type only. Furthermore, prognosis-predictive genes, as well as potential processes and pathways, were significantly among the anatomical location-dependent types that the genes barely overlapped.
Conclusions: There are disparities in almost all aspects among distal, intrahepatic, and perihilar CCAs. Anatomical location needs to be considered in treatment, prognostic estimation, identifying targets, and developing therapeutic approaches for CCA.
Keywords: Biliary malignancy; bile duct cancer; distal cholangiocarcinoma (dCCA); intrahepatic cholangiocarcinoma (iCCA); perihilar cholangiocarcinoma (hiCCA).
2019 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures



Comment in
-
New era with the genetic assessment for biliary tree cancers beyond the anatomical assessment alone.Ann Transl Med. 2020 Jun;8(12):732. doi: 10.21037/atm.2020.03.206. Ann Transl Med. 2020. PMID: 32647657 Free PMC article. No abstract available.
-
Cholangiocarcinoma: three different entities based on location.Ann Transl Med. 2020 Jun;8(12):738. doi: 10.21037/atm.2020.03.167. Ann Transl Med. 2020. PMID: 32647663 Free PMC article. No abstract available.
References
-
- Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2016;13:261-80. 10.1038/nrgastro.2016.51 - DOI - PubMed
LinkOut - more resources
Full Text Sources
