Retinal analysis of a mouse model of Alzheimer's disease with multicontrast optical coherence tomography
- PMID: 32042855
- PMCID: PMC6999077
- DOI: 10.1117/1.NPh.7.1.015006
Retinal analysis of a mouse model of Alzheimer's disease with multicontrast optical coherence tomography
Abstract
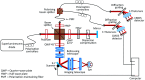
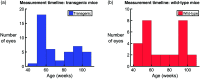
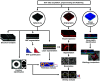
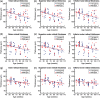
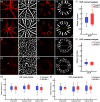
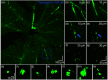
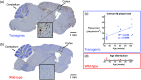
Significance. Recent Alzheimer's disease (AD) patient studies have focused on retinal analysis, as the retina is the only part of the central nervous system that can be imaged noninvasively by optical methods. However, as this is a relatively new approach, the occurrence and role of retinal pathological features are still debated. Aim. The retina of an APP/PS1 mouse model was investigated using multicontrast optical coherence tomography (OCT) in order to provide a documentation of what was observed in both transgenic and wild-type mice. Approach. Both eyes of 24 APP/PS1 transgenic mice (age: 45 to 104 weeks) and 15 age-matched wild-type littermates were imaged by the custom-built OCT system. At the end of the experiment, retinas and brains were harvested from a subset of the mice (14 transgenic, 7 age-matched control) in order to compare the in vivo results to histological analysis and to quantify the cortical amyloid beta plaque load. Results. The system provided a combination of standard reflectivity data, polarization-sensitive data, and OCT angiograms. Qualitative and quantitative information from the resultant OCT images was extracted on retinal layer thickness and structure, presence of hyper-reflective foci, phase retardation abnormalities, and retinal vasculature. Conclusions. Although multicontrast OCT revealed abnormal structural properties and phase retardation signals in the retina of this APP/PS1 mouse model, the observations were very similar in transgenic and control mice.
Keywords: Alzheimer’s disease; amyloid beta; histology; optical coherence tomography; polarization; retina.
© The Authors. Published by SPIE under a Creative Commons Attribution 4.0 Unported License. Distribution or reproduction of this work in whole or in part requires full attribution of the original publication, including its DOI.
Figures




References
-
- Alzheimer’s Association, “2019 Alzheimer’s disease facts and figures,” Alzheimers Dement. 15, 321–387 (2019).10.1016/j.jalz.2019.01.010 - DOI
-
- Hurtado-Puerto A. M., Russo C., Fregni F., Alzheimer’s Disease, pp. 297–338, Springer, New York: (2018).
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

