Down-regulation of argininosuccinate lyase induces hepatoma cell apoptosis through activating Bax signaling pathway
- PMID: 32042869
- PMCID: PMC6997574
- DOI: 10.1016/j.gendis.2018.11.003
Down-regulation of argininosuccinate lyase induces hepatoma cell apoptosis through activating Bax signaling pathway
Abstract
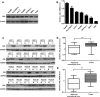
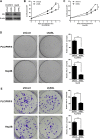
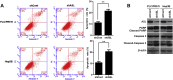
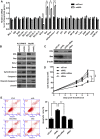
Argininosuccinate lyase (ASL) plays an important role in the hepatic urea cycle, and can catalyze the reversible reaction of argininosuccinate to arginine and fumarate. However, the function of ASL in hepatocellular carcinoma (HCC) is not fully understood. In this study, we found that ASL expression was frequently upregulated in HCC tissues and HCC cell lines. Knock down of ASL inhibited cell proliferation and induced apoptosis in HCC cells. Mechanistic studies revealed the BCL2-associated X protein (Bax) signaling pathway which determines cancer cell apoptosis was regulated by ASL. Moreover, the depletion of Bax restored the inhibition of cell growth and reduced apoptosis initiated by ASL silencing. Together, the study demonstrated that ASL regulated HCC cell growth and apoptosis by modulating Bax signaling. Thus, the therapeutic targeting of ASL may offer options for HCC treatment.
Keywords: ASL; Apoptosis; Bax; HCC; Proliferation.
© 2018 Chongqing Medical University. Production and hosting by Elsevier B.V.
Figures
References
-
- Budny A., Kozlowski P., Kaminska M. Epidemiology and risk factors of hepatocellular carcinoma. Polski merkuriusz lekarski – Organ Polskiego Towarzystwa Lekarskiego. 2017;43(255):133–139. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

