Environmental and genetic determinants of plasmid mobility in pathogenic Escherichia coli
- PMID: 32042895
- PMCID: PMC6981087
- DOI: 10.1126/sciadv.aax3173
Environmental and genetic determinants of plasmid mobility in pathogenic Escherichia coli
Abstract
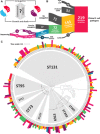
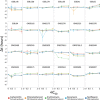
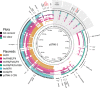
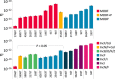
Plasmids are key vehicles of horizontal gene transfer (HGT), mobilizing antibiotic resistance, virulence, and other traits among bacterial populations. The environmental and genetic forces that drive plasmid transfer are poorly understood, however, due to the lack of definitive quantification coupled with genomic analysis. Here, we integrate conjugative phenotype with plasmid genotype to provide quantitative analysis of HGT in clinical Escherichia coli pathogens. We find a substantial proportion of these pathogens (>25%) able to readily spread resistance to the most common classes of antibiotics. Antibiotics of varied modes of action had less than a 5-fold effect on conjugation efficiency in general, with one exception displaying 31-fold promotion upon exposure to macrolides and chloramphenicol. In contrast, genome sequencing reveals plasmid incompatibility group strongly correlates with transfer efficiency. Our findings offer new insights into the determinants of plasmid mobility and have implications for the development of treatments that target HGT.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures

Similar articles
-
Dissecting horizontal and vertical gene transfer of antibiotic resistance plasmid in bacterial community using microfluidics.Environ Int. 2019 Oct;131:105007. doi: 10.1016/j.envint.2019.105007. Epub 2019 Jul 18. Environ Int. 2019. PMID: 31326825
-
Genome Analysis of the Carbapenem- and Colistin-Resistant Escherichia coli Isolate NRZ14408 Reveals Horizontal Gene Transfer Pathways towards Panresistance and Enhanced Virulence.Antimicrob Agents Chemother. 2017 Mar 24;61(4):e02359-16. doi: 10.1128/AAC.02359-16. Print 2017 Apr. Antimicrob Agents Chemother. 2017. PMID: 28096159 Free PMC article. No abstract available.
-
Antibiotic resistance correlates with transmission in plasmid evolution.Evolution. 2014 Dec;68(12):3368-80. doi: 10.1111/evo.12537. Epub 2014 Nov 24. Evolution. 2014. PMID: 25351426
-
Triclosan at environmentally relevant concentrations promotes horizontal transfer of multidrug resistance genes within and across bacterial genera.Environ Int. 2018 Dec;121(Pt 2):1217-1226. doi: 10.1016/j.envint.2018.10.040. Epub 2018 Oct 30. Environ Int. 2018. PMID: 30389380
-
Towards a better understanding of antimicrobial resistance dissemination: what can be learnt from studying model conjugative plasmids?Mil Med Res. 2022 Jan 10;9(1):3. doi: 10.1186/s40779-021-00362-z. Mil Med Res. 2022. PMID: 35012680 Free PMC article. Review.
Cited by
-
Duplicated antibiotic resistance genes reveal ongoing selection and horizontal gene transfer in bacteria.Nat Commun. 2024 Feb 16;15(1):1449. doi: 10.1038/s41467-024-45638-9. Nat Commun. 2024. PMID: 38365845 Free PMC article.
-
Estimating the transfer rates of bacterial plasmids with an adapted Luria-Delbrück fluctuation analysis.PLoS Biol. 2022 Jul 25;20(7):e3001732. doi: 10.1371/journal.pbio.3001732. eCollection 2022 Jul. PLoS Biol. 2022. PMID: 35877684 Free PMC article.
-
Functions predict horizontal gene transfer and the emergence of antibiotic resistance.Sci Adv. 2021 Oct 22;7(43):eabj5056. doi: 10.1126/sciadv.abj5056. Epub 2021 Oct 22. Sci Adv. 2021. PMID: 34678056 Free PMC article.
-
Horizontal gene transfer enables programmable gene stability in synthetic microbiota.Nat Chem Biol. 2022 Nov;18(11):1245-1252. doi: 10.1038/s41589-022-01114-3. Epub 2022 Sep 1. Nat Chem Biol. 2022. PMID: 36050493 Free PMC article.
-
Vertical and horizontal gene transfer tradeoffs direct plasmid fitness.Mol Syst Biol. 2023 Feb 10;19(2):e11300. doi: 10.15252/msb.202211300. Epub 2022 Dec 27. Mol Syst Biol. 2023. PMID: 36573357 Free PMC article.
References
-
- J. O’Neill, Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations (Review on Antimicrobial Resistance, 2014), pp. 1–16 .
-
- M. Barlow, in Horizontal Gene Transfer: Genomes in Flux, B. G. Maria, J. P. Gogarten, L. Olendzenski, Eds. (Humana Press, 2009), vol. 532, pp. 397–411.
-
- D’Costa V. M., McGrann K. M., Hughes D. W., Wright G. D., Sampling the antibiotic resistome. Science 311, 374–377 (2006). - PubMed
-
- Graf F. E., Palm M., Warringer J., Farewell A., Inhibiting conjugation as a tool in the fight against antibiotic resistance. Drug Dev. Res. 80, 19–23 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

