A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice
- PMID: 32043464
- PMCID: PMC7012599
- DOI: 10.7554/eLife.53111
A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice
Abstract
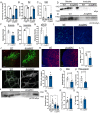
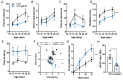
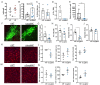
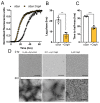
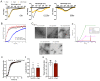
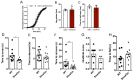
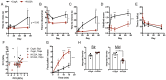
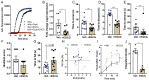
Amyloids are a class of protein with unique self-aggregation properties, and their aberrant accumulation can lead to cellular dysfunctions associated with neurodegenerative diseases. While genetic and environmental factors can influence amyloid formation, molecular triggers and/or facilitators are not well defined. Growing evidence suggests that non-identical amyloid proteins may accelerate reciprocal amyloid aggregation in a prion-like fashion. While humans encode ~30 amyloidogenic proteins, the gut microbiome also produces functional amyloids. For example, curli are cell surface amyloid proteins abundantly expressed by certain gut bacteria. In mice overexpressing the human amyloid α-synuclein (αSyn), we reveal that colonization with curli-producing Escherichia coli promotes αSyn pathology in the gut and the brain. Curli expression is required for E. coli to exacerbate αSyn-induced behavioral deficits, including intestinal and motor impairments. Purified curli subunits accelerate αSyn aggregation in biochemical assays, while oral treatment of mice with a gut-restricted amyloid inhibitor prevents curli-mediated acceleration of pathology and behavioral abnormalities. We propose that exposure to microbial amyloids in the gastrointestinal tract can accelerate αSyn aggregation and disease in the gut and the brain.
Keywords: Curli; E. coli; alpha-synuclein; infectious disease; microbiology; microbiome; mouse; neuroscience.
© 2020, Sampson et al.
Conflict of interest statement
TS has intellectual property pending in relationship to the content of this manuscript, US Patent App. 15/893,456 and 16/302,321. CC, NJ, AM, ML, GS, TT, BN, IH, JD, SJ, RK, PW, VG No competing interests declared, MC a member of the Scientific Advisory Board of Axial Biotherapeutics. SM has financial interest in Axial Biotherapeutics. Has intellectual property pending in relationship to the content of this manuscript, US Patent App. 15/893,456 and 16/302,321.
Figures



References
-
- Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S, Ferri V, Cancello R, Ceccarani C, Faierman S, Pinelli G, Bellis G, Zecca L, Cereda E, Consolandi C, Pezzoli G. Unraveling gut Microbiota in Parkinson's disease and atypical parkinsonism. Movement Disorders. 2019;34:396–405. doi: 10.1002/mds.27581. - DOI - PubMed
-
- Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, Zabari M, Brik RB-Z, Kviatcovsky D, Zmora N, Cohen Y, Bar N, Levi I, Amar N, Mehlman T, Brandis A, Biton I, Kuperman Y, Tsoory M, Alfahel L, Harmelin A, Schwartz M, Israelson A, Arike L, Johansson MEV, Hansson GC, Gotkine M, Segal E, Elinav E. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474–480. doi: 10.1038/s41586-019-1443-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

