The Safety and Efficacy of Checkpoint Inhibitors in Transplant Recipients: A Case Series and Systematic Review of Literature
- PMID: 32043699
- PMCID: PMC7288631
- DOI: 10.1634/theoncologist.2019-0659
The Safety and Efficacy of Checkpoint Inhibitors in Transplant Recipients: A Case Series and Systematic Review of Literature
Abstract
Limited data exist on safety and efficacy of immune checkpoint inhibitors (ICIs) among organ transplant recipients. The objective of this study was to report a case series of two patients with renal transplant who received treatment with an ICI and to conduct a pooled analysis of published cases to describe the safety and efficacy of ICIs in organ transplant patients. A systematic search in the Google Scholar and PubMed databases was carried out to include all the published cases of organ transplant patients who received treatment with ICIs including programmed cell death protein 1 (PD-1), programmed death-ligand 1, or cytotoxic lymphocyte antigen-4 inhibitors since their inscription to January 31, 2019. In the present series of two cases with renal allografts who received pembrolizumab, one patient with squamous cell carcinoma of the skin experienced complete response (CR), whereas another patient with melanoma had a mixed response. Both patients experienced allograft rejection, but graft was salvaged. The pooled analysis of 64 patients published in literature showed that overall allograft rejection rate is 41% in organ transplant recipients following ICI therapy. The graft rejection rate was 44% (17/39) for renal, 39% (7/19) for liver, and 20% (1/5) for cardiac allografts. The highest risk was seen among patients who were treated with PD-1 inhibitors, 20/42 (48%)-13/24 (54%) on nivolumab and 7/18 (39%) on pembrolizumab. The risk was lowest with ipilimumab, 23% (3/13). The overall response rate (CR + partial response [PR]) was 20% with ipilimumab, 26% with nivolumab, and 53% with pembrolizumab, whereas disease control rate (CR + PR + stable disease) was 35% with ipilimumab, 37% with nivolumab, and 53% with pembrolizumab. None of the variables including age, gender, type of cancer, type of allograft, type of immunosuppression, time since transplantation to initiation of ICI, and prior history of rejection were significantly associated with the transplant rejection on univariate analysis. The efficacy of ICI among patients with organ transplant appears promising, warranting testing in prospective clinical trials. The risk of rejection and allograft loss is considerable; therefore, the risk and alternative form of therapies should be thoroughly discussed with the transplant patients prior to initiating ICI therapy. IMPLICATIONS FOR PRACTICE: Transplant recipients are at higher risk of developing cancers. Although immune checkpoint inhibitors have been shown to improve the outcome in more than one cancer type, transplant recipients were excluded from these trials. Most of the data on the safety and efficacy of immune checkpoint inhibitors in transplant patients are based upon case series and case reports. The pooled data from these reports suggest that anti-programmed death-ligand 1 inhibitors have reasonable safety and efficacy among organ transplant patients, which warrants testing in clinical trials.
Keywords: Heart transplant; Immune checkpoint inhibitors; Liver transplant; Renal transplant; Transplant rejection.
© AlphaMed Press 2020.
Conflict of interest statement
Figures


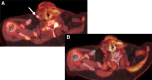
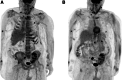
References
-
- Ipilimumab [package insert]. Princeton, NJ: Bristol‐Myers Squibb Company; 2018.
-
- Durvalumab [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2018.
-
- Pembrolizumab [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2018.
-
- Atezolizumab [package insert]. South San Francisco, CA: Genentech, Inc.; 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

