Streptococcus mutans SpxA2 relays the signal of cell envelope stress from LiaR to effectors that maintain cell wall and membrane homeostasis
- PMID: 32043713
- PMCID: PMC7202993
- DOI: 10.1111/omi.12282
Streptococcus mutans SpxA2 relays the signal of cell envelope stress from LiaR to effectors that maintain cell wall and membrane homeostasis
Abstract
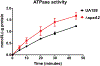
Streptococcus mutans is a major etiologic agent of dental caries, which is the most common chronic infectious disease worldwide. S. mutans is particularly adept at causing caries due to its exceptional capacity to form biofilms and its ability to survive acidic conditions that arrest acid production and growth in many more benign members of the oral microbiota. Two mechanisms utilized by S. mutans to tolerate acid are: modulation of the membrane fatty acid content and utilization of the F1 F0 -ATPase to pump protons out of the cytosol. In this study, the role of the spxA2 transcriptional regulator in these two pathways, and overall cell envelope homeostasis, was examined. Loss of spxA2 resulted in an increase in the proportion of saturated fatty acids in the S. mutans membrane and altered transcription of several genes involved in the production of these membrane fatty acids, including fabT and fabM. Furthermore, activity of the F1 F0 -ATPase was increased in the ∆spxA2 strain. Transcription of spxA2 was elevated in the presence of a variety of membrane stressors, and highly dependent on the liaR component of the LiaFSR system, which is known to sense cell envelope stress in many Gram-positive bacteria. Finally, deletion of ∆spxA2 led to altered susceptibility of S. mutans to membrane stressors. Overall, the results of this study indicate that spxA2 serves a crucial role in transmitting the signal of cell wall/membrane damage from the LiaFSR sensor to downstream effectors in the SpxA2 regulon which restore and maintain membrane and cell wall homeostasis.
Keywords: Streptococcus mutans; Spx; dental caries; environmental regulation; fatty acids.
© 2020 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures


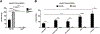
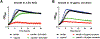
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

