Health-Related Quality of Life in MONARCH 2: Abemaciclib plus Fulvestrant in Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer After Endocrine Therapy
- PMID: 32043763
- PMCID: PMC7011625
- DOI: 10.1634/theoncologist.2019-0551
Health-Related Quality of Life in MONARCH 2: Abemaciclib plus Fulvestrant in Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer After Endocrine Therapy
Abstract
Background: In the phase III MONARCH 2 study (NCT02107703), abemaciclib plus fulvestrant significantly improved progression-free survival (PFS) versus placebo plus fulvestrant in patients with hormone receptor-positive (HR+), HER2-negative advanced breast cancer (ABC). This study assessed patient-reported pain, global health-related quality of life (HRQoL), functioning, and symptoms.
Materials and methods: Abemaciclib or placebo (150 p.o. mg twice daily) plus fulvestrant (500 mg, per label) were randomly assigned (2:1). The modified Brief Pain Inventory, Short Form (mBPI-sf); European Organization for Research and Treatment of Cancer (EORTC) QoL Core 30 (QLQ-C30); and Breast Cancer Questionnaire (QLQ-BR23) assessed outcomes. Data were collected at baseline, cycle 2, every two cycles 3-13, thereafter at every three cycles, and 30 days postdiscontinuation. Longitudinal mixed regression and Cox proportional hazards models assessed postbaseline change and time to sustained deterioration (TTSD) by study arm.
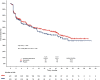
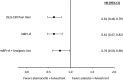
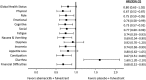
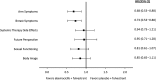
Results: On-treatment HRQoL scores were consistently maintained from baseline and similar between arms. Patients in the abemaciclib arm (n = 446) experienced a 4.9-month delay in pain deterioration (mBPI-sf), compared with the control arm (n = 223), and significantly greater TTSD on the mBPI-sf and analgesic use (hazard ratio, 0.76; 95% CI, 0.59-0.98) and QLQ-C30 pain item (hazard ratio, 0.62; 95% CI, 0.48-0.79). TTSD for functioning and most symptoms significantly favored the abemaciclib arm, including fatigue, nausea and vomiting, and cognitive and social functioning. Only diarrhea significantly favored the control arm (hazard ratio, 1.60; 95% CI, 1.20-2.10).
Conclusion: HRQoL was maintained on abemaciclib plus fulvestrant. Alongside superior PFS and manageable safety profile, results support treatment with abemaciclib plus fulvestrant in a population of patients with endocrine-resistant HR+, HER2-negative ABC.
Implications for practice: In MONARCH 2, abemaciclib plus fulvestrant demonstrated superior efficacy and a manageable safety profile for patients with in hormone receptor-positive (HR+), HER2-negative (-) advanced breast cancer (ABC). Impact on health-related quality of life (HRQoL) is important to consider, given the palliative nature of ABC treatment. In this study, abemaciclib plus fulvestrant, compared with placebo plus fulvestrant, significantly delayed sustained deterioration of pain and other patient-reported symptoms (including fatigue, nausea, vomiting), and social and cognitive functioning. Combined with demonstrated clinical benefit and tolerability, the stabilization of patient-reported symptoms and HRQoL further supports abemaciclib plus fulvestrant as a desirable treatment option in endocrine resistant, HR+, HER2- ABC.
Keywords: Abemaciclib; Advanced breast cancer; Patient-reported outcomes; Quality of life.
© 2019 Eli Lilly and Company. The Oncologist published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
Figures
References
-
- Patnaik A, Rosen LS, Tolaney SM et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non‐small cell lung cancer, and other solid tumors. Cancer Discov 2016;6:740–753. - PubMed
-
- Sledge GW Jr, Toi M, Neven P et al. MONARCH2: Abemaciclib in combination with fulvestrant in women with HR+/HER2‐ advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–2884. - PubMed
-
- Lallena MJ, Boehnke K, Torres R et al. In‐vitro characterization of abemaciclib pharmacology in ER+ breast cancer cell lines. Cancer Res 2015;75(15 suppl):3101a.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

