The European Medicines Agency Review of Kymriah (Tisagenlecleucel) for the Treatment of Acute Lymphoblastic Leukemia and Diffuse Large B-Cell Lymphoma
- PMID: 32043764
- PMCID: PMC7011647
- DOI: 10.1634/theoncologist.2019-0233
The European Medicines Agency Review of Kymriah (Tisagenlecleucel) for the Treatment of Acute Lymphoblastic Leukemia and Diffuse Large B-Cell Lymphoma
Abstract
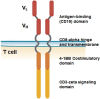
Chimeric antigen receptor (CAR)-engineered T-cell therapy is becoming one of the most promising approaches in the treatment of cancer. On June 28, 2018, the Committee for Advanced Therapies (CAT) and the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Kymriah for pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse after transplant, or in second or later relapse and for adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy. Kymriah became one of the first European Union-approved CAR T therapies. The active substance of Kymriah is tisagenlecleucel, an autologous, immunocellular cancer therapy that involves reprogramming the patient's own T cells to identify and eliminate CD19-expressing cells. This is achieved by addition of a transgene encoding a CAR. The benefit of Kymriah was its ability to achieve remission with a significant duration in patients with ALL and an objective response with a significant duration in patients with DLBCL. The most common hematological toxicity was cytopenia in both patients with ALL and those with DLBCL. Nonhematological side effects in patients with ALL were cytokine release syndrome (CRS), infections, secondary hypogammaglobulinemia due to B-cell aplasia, pyrexia, and decreased appetite. The most common nonhematological side effects in patients with DLBCL were CRS, infections, pyrexia, diarrhea, nausea, hypotension, and fatigue. Kymriah also received an orphan designation on April 29, 2014, following a positive recommendation by the Committee for Orphan Medicinal Products (COMP). Maintenance of the orphan designation was recommended at the time of marketing authorization as the COMP considered the product was of significant benefit for patients with both conditions. IMPLICATIONS FOR PRACTICE: Chimeric antigen receptor (CAR)-engineered T-cell therapy is becoming the most promising approach in cancer treatment, involving reprogramming the patient's own T cells with a CAR-encoding transgene to identify and eliminate cancer-specific surface antigen-expressing cells. On June 28, 2018, Kymriah became one of the first EMA approved CAR T therapies. CAR T technology seems highly promising for diseases with single genetic/protein alterations; however, for more complex diseases there will be challenges to target clonal variability within the tumor type or clonal evolution during disease progression. Products with a lesser toxicity profile or more risk-minimization tools are also anticipated.
Keywords: Acute lymphoblastic leukemia; Chimeric antigen receptor; Cytokine release syndrome; Diffuse large B-cell lymphoma; Kymriah (Tisagenlecleucel, CTL019); Replication-competent lentivirus.
© AlphaMed Press 2019.
Conflict of interest statement
Figures

Similar articles
-
EMA Review of Axicabtagene Ciloleucel (Yescarta) for the Treatment of Diffuse Large B-Cell Lymphoma.Oncologist. 2020 Oct;25(10):894-902. doi: 10.1634/theoncologist.2019-0646. Epub 2020 Apr 27. Oncologist. 2020. PMID: 32339368 Free PMC article. Review.
-
[Eligibility of patients for CAR T-cell: Expert opinion-based collaborative work by the SFGM-TC].Bull Cancer. 2021 Jul-Aug;108(7-8):725-729. doi: 10.1016/j.bulcan.2020.10.017. Epub 2021 Jan 7. Bull Cancer. 2021. PMID: 33423776 Review. French.
-
Determinants of outcomes and advances in CD19-directed chimeric antigen receptor therapy for B-cell acute lymphoblastic leukemia.Eur J Haematol. 2024 Jan;112(1):51-63. doi: 10.1111/ejh.14132. Eur J Haematol. 2024. PMID: 38105391 Review.
-
Tisagenlecleucel in Acute Lymphoblastic Leukemia: A Review of the Literature and Practical Considerations.Ann Pharmacother. 2021 Apr;55(4):466-479. doi: 10.1177/1060028020948165. Epub 2020 Aug 7. Ann Pharmacother. 2021. PMID: 32762363 Review.
-
Kymriah® (tisagenlecleucel) - An overview of the clinical development journey of the first approved CAR-T therapy.Hum Vaccin Immunother. 2023 Dec 31;19(1):2210046. doi: 10.1080/21645515.2023.2210046. Epub 2023 May 15. Hum Vaccin Immunother. 2023. PMID: 37185251 Free PMC article.
Cited by
-
Anti-Cancer Potential of Transiently Transfected HER2-Specific Human Mixed CAR-T and NK Cell Populations in Experimental Models: Initial Studies on Fucosylated Chondroitin Sulfate Usage for Safer Treatment.Biomedicines. 2023 Sep 18;11(9):2563. doi: 10.3390/biomedicines11092563. Biomedicines. 2023. PMID: 37761005 Free PMC article.
-
CAR-T-Cell Therapy in Multiple Myeloma: B-Cell Maturation Antigen (BCMA) and Beyond.Vaccines (Basel). 2023 Nov 16;11(11):1721. doi: 10.3390/vaccines11111721. Vaccines (Basel). 2023. PMID: 38006053 Free PMC article. Review.
-
Unlocking the potential: advancements and applications of gene therapy in severe disorders.Ann Med. 2025 Dec;57(1):2516697. doi: 10.1080/07853890.2025.2516697. Epub 2025 Jun 17. Ann Med. 2025. PMID: 40526097 Free PMC article. Review.
-
Establishing Rationale for the Clinical Development of Cell Therapy Products: Consensus between Risk and Benefit.Int J Stem Cells. 2023 Feb 28;16(1):16-26. doi: 10.15283/ijsc21189. Epub 2022 Dec 31. Int J Stem Cells. 2023. PMID: 36581365 Free PMC article. Review.
-
Successful treatment of acute B lymphoblastic leukemia relapse in the skin and testicle by anti-CD19 CAR-T with IL-6 knocking down: a case report.Biomark Res. 2020 May 6;8:12. doi: 10.1186/s40364-020-00193-5. eCollection 2020. Biomark Res. 2020. PMID: 32399214 Free PMC article.
References
-
- Möricke A, Zimmermann M, Reiter A et al. Long‐term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL‐BFM study group from 1981 to 2000. Leukemia 2010;24:265–284. - PubMed
-
- Locatelli F, Martin M, Bernardo ME et al. How I treat relapsed childhood acute lymphoblastic leukemia. Blood 2012;120:2807–2816. - PubMed
-
- Tallen G, Ratei R, Mann G et al. Long‐term outcome in children with relapsed acute lymphoblastic leukemia after time‐point and site‐of‐relapse stratification and intensified short‐course multidrug chemotherapy: Results of trial ALL‐REZ BFM 90. J Clin Oncol 2010;28:2339–2347. - PubMed
-
- Bailey LC, Lange BJ, Rheingold SR et al. Bone‐marrow relapse in paediatric acute lymphoblastic leukaemia. Lancet Oncol 2008;9:873–883. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

