De Novo Versus Recurrent HER2-Positive Metastatic Breast Cancer: Patient Characteristics, Treatment, and Survival from the SystHERs Registry
- PMID: 32043771
- PMCID: PMC7011632
- DOI: 10.1634/theoncologist.2019-0446
De Novo Versus Recurrent HER2-Positive Metastatic Breast Cancer: Patient Characteristics, Treatment, and Survival from the SystHERs Registry
Abstract
Background: Limited data exist describing real-world treatment of de novo and recurrent HER2-positive metastatic breast cancer (MBC).
Materials and methods: The Systemic Therapies for HER2-Positive Metastatic Breast Cancer Study (SystHERs) was a fully enrolled (2012-2016), observational, prospective registry of patients with HER2-positive MBC. Patients aged ≥18 years and ≤6 months from HER2-positive MBC diagnosis were treated and assessed per their physician's standard practice. The primary endpoint was to characterize treatment patterns by de novo versus recurrent MBC status, compared descriptively. Secondary endpoints included patient characteristics, progression-free and overall survival (PFS and OS, by Kaplan-Meier method; hazard ratio [HR] and 95% confidence interval [CI] by Cox regression), and patient-reported outcomes.
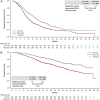
Results: Among 977 eligible patients, 49.8% (n = 487) had de novo and 50.2% (n = 490) had recurrent disease. A higher proportion of de novo patients had hormone receptor-negative disease (34.9% vs. 24.9%), bone metastasis (57.1% vs. 45.9%), and/or liver metastasis (41.9% vs. 33.1%), and a lower proportion had central nervous system metastasis (4.3% vs. 13.5%). De novo patients received first-line regimens containing chemotherapy (89.7%), trastuzumab (95.7%), and pertuzumab (77.8%) more commonly than recurrent patients (80.0%, 85.9%, and 68.6%, respectively). De novo patients had longer median PFS (17.7 vs. 11.9 months; HR, 0.69; 95% CI, 0.59-0.80; p < .0001) and OS (not estimable vs. 44.5 months; HR, 0.55; 95% CI, 0.44-0.69; p < .0001).
Conclusion: Patients with de novo versus recurrent HER2-positive MBC exhibit different disease characteristics and survival durations, suggesting these groups have distinct outcomes. These differences may affect future clinical trial design. Clinical trial identification number. NCT01615068 (clinicaltrials.gov).
Implications for practice: SystHERs was an observational registry of patients with HER2-positive metastatic breast cancer (MBC), which is a large, modern, real-world data set for this population and, thereby, provides a unique opportunity to study patients with de novo and recurrent HER2-positive MBC. In SystHERs, patients with de novo disease had different baseline demographics and disease characteristics, had superior clinical outcomes, and more commonly received first-line chemotherapy and/or trastuzumab versus those with recurrent disease. Data from this and other studies suggest that de novo and recurrent MBC have distinct outcomes, which may have implications for disease management strategies and future clinical study design.
Keywords: De novo; HER2-positive metastatic breast cancer; Recurrent; Registry; SystHERs.
© 2019 The Authors. The Oncologist published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous