Impact of Baseline Nutrition and Exercise Status on Toxicity and Outcomes in Phase I and II Oncology Clinical Trial Participants
- PMID: 32043776
- PMCID: PMC7011642
- DOI: 10.1634/theoncologist.2019-0289
Impact of Baseline Nutrition and Exercise Status on Toxicity and Outcomes in Phase I and II Oncology Clinical Trial Participants
Abstract
Background: Malnutrition and physical inactivity are common in patients with advanced cancer and are associated with poor outcomes. There are increasing data that altered body composition is related to the pharmacokinetic properties of cancer therapies. These adverse conditions may impact outcomes in early-phase oncology clinical trials.
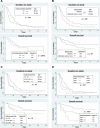
Materials and methods: We aimed to understand the relationships between baseline nutrition and exercise status with important trial endpoints including treatment-related toxicity and survival. Baseline assessments of nutrition and exercise status were conducted in patients prior to initiation of phase I and II oncology clinical trials. Patients were followed prospectively for the onset of adverse events. Tumor response and survival data were also obtained. Fisher's exact test and chi-square analysis were used to determine statistical significance. Kaplan-Meier curves were used to compare patient duration on study and survival.
Results: One hundred patients were recruited, of whom 87 were initiating a phase I trial. Sixty percent were initiating trials studying immunotherapeutic agents. Critical malnutrition was found in 39% of patients, and 52% were sedentary. Patients who were malnourished had significantly increased rates of grade ≥ 3 toxicity (p = .001), hospitalizations (p = .001), and inferior disease control rate (p = .019). Six-month overall survival was significantly reduced in malnourished patients versus nonmalnourished patients (47% vs. 84%; p = .0003), as was median duration on study (48 days vs. 105 days; p = .047). Being sedentary at baseline was associated with decreased duration on study (57 days vs. 105 days; p = .019).
Conclusion: Malnutrition and sedentary lifestyle are highly prevalent in patients enrolling on early-phase oncology clinical trials and are associated with poor outcomes. The quality of data from these studies may be compromised as a result of these pre-existing conditions.
Implications for practice: Phase I and II trials are critical steps in the development of effective cancer therapeutics, yet only a small percentage of agents are ultimately approved for human cancer care. Despite increasing awareness of the interactions between malnutrition, sarcopenia, and treatment-related outcomes such as toxicity and response, these factors are not commonly incorporated into therapeutic decision making at the time of clinical trial consideration. Nutritional status and physical performance may be key biomarkers of mechanisms mediating treatment-related toxicity, dose modifications, risk of hospitalizations, and success of novel agents. This study advocates that a baseline nutritional assessment and early nutritional support may improve tolerability and response to experimental therapies.
Keywords: Cachexia; Exercise; Malnutrition; Outcomes; Toxicity.
© AlphaMed Press 2019.
Conflict of interest statement
Figures
References
-
- Pond GR, Siu LL, Moore M et al. Nomograms to predict serious adverse events in phase II clinical trials of molecularly targeted agents. J Clin Oncol 2008;26:1324–1330. - PubMed
-
- Laviano A, Meguid MM, Rossi‐Fanelli F. Cancer anorexia: Clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol 2003;4:686–694. - PubMed
-
- Fearon K, Strasser F, Anker SD et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011;12:489–495. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

