Hyperprogression and Immune Checkpoint Inhibitors: Hype or Progress?
- PMID: 32043794
- PMCID: PMC7011624
- DOI: 10.1634/theoncologist.2019-0636
Hyperprogression and Immune Checkpoint Inhibitors: Hype or Progress?
Abstract
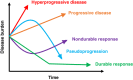
There are currently seven approved immune checkpoint inhibitors (ICIs) for the treatment of various cancers. These drugs are associated with profound, durable responses in a subset of patients with advanced cancers. Unfortunately, in addition to individuals whose tumors show resistance, there is a minority subgroup treated with ICIs who demonstrate a paradoxical acceleration in the rate of growth or their tumors-hyperprogressive disease. Hyperprogressive disease is associated with significantly worse outcomes in these patients. This phenomenon, though still a matter of dispute, has been recognized by multiple groups of investigators across the globe and in diverse types of cancers. There are not yet consensus standardized criteria for defining hyperprogressive disease, but most commonly time to treatment failure less than 2 months and an increase in pace of progression of at least twofold between pre-immunotherapy and on-treatment imaging has been used. In some patients, the change in rate of progression can be especially dramatic-up to 35- to 40-fold. MDM2 amplification and EGFR mutations have been suggested as genomic correlates of increased risk of hyperprogression, but these correlates require validation. The underlying mechanism for hyperprogression is not known but warrants urgent investigation.
Keywords: Cancer clinical trials; Hyperprogressive disease; Immune checkpoint inhibitors; Immunotherapy.
© 2019 The Authors. The Oncologist published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
Figures
References
-
- Marcus L, Lemery SJ, Keegan P et al. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability‐high solid tumors. Clin Cancer Res 2019;25:3753–3758. - PubMed
-
- Nivolumab approved for lung cancer . Cancer Discov 2015;5:OF1. - PubMed
-
- Kim ES. Avelumab: First global approval. Drugs 2017;77:929–937. - PubMed
-
- Weinstock C, Khozin S, Suzman D et al. U.S. Food and Drug Administration approval summary: Atezolizumab for metastatic non‐small cell lung cancer. Clin Cancer Res 2017;23:4534–4539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

