Multicomponent Pyrazole Synthesis from Alkynes, Nitriles, and Titanium Imido Complexes via Oxidatively Induced N-N Bond Coupling
- PMID: 32043879
- PMCID: PMC7201868
- DOI: 10.1021/jacs.9b13173
Multicomponent Pyrazole Synthesis from Alkynes, Nitriles, and Titanium Imido Complexes via Oxidatively Induced N-N Bond Coupling
Abstract
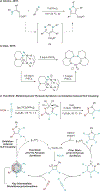
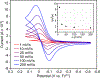
Pyrazoles are an important class of heterocycles found in a wide range of bioactive compounds and pharmaceuticals. Pyrazole synthesis often requires hydrazine or related reagents where an intact N-N bond is conservatively installed into a pyrazole precursor fragment. Herein, we report the multicomponent oxidative coupling of alkynes, nitriles, and Ti imido complexes for the synthesis of multisubstituted pyrazoles. This modular method avoids potentially hazardous reagents like hydrazine, instead forming the N-N bond in the final step via oxidation-induced coupling on Ti. The mechanism of this transformation has been studied in-depth through stoichiometric reactions of the key diazatitanacyclohexadiene intermediate, which can be accessed via multicomponent coupling of Ti imidos with nitriles and alkynes, ring opening of 2-imino-2H-azirines, or direct metalation of 4-azadiene-1-amine derivatives. The critical transformation in this reaction is the 2-electron oxidation-induced N-N coupling on Ti. This is a rare example of formal N-N coupling on a metal center, which likely occurs through an electrocyclic mechanism analogous to a Nazarov cyclization. Conveniently, these 2-electron-oxidized diazatitanacyclohexadiene intermediates can be accessed via disproportionation of the 1-electron-oxidized species, which allows utilization of weak oxidants such as TEMPO.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





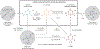
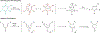
Similar articles
-
Ti-Catalyzed and -Mediated Oxidative Amination Reactions.Acc Chem Res. 2021 Sep 7;54(17):3476-3490. doi: 10.1021/acs.accounts.1c00368. Epub 2021 Aug 22. Acc Chem Res. 2021. PMID: 34420307 Free PMC article.
-
Single-step synthesis of pyrazoles using titanium catalysis.Chem Commun (Camb). 2012 Jan 11;48(3):440-2. doi: 10.1039/c1cc15809k. Epub 2011 Nov 14. Chem Commun (Camb). 2012. PMID: 22083455
-
Titanium-catalyzed multicomponent couplings: efficient one-pot syntheses of nitrogen heterocycles.Acc Chem Res. 2015 Nov 17;48(11):2822-33. doi: 10.1021/acs.accounts.5b00280. Epub 2015 Aug 21. Acc Chem Res. 2015. PMID: 26295382
-
Gold α-oxo carbenoids in catalysis: catalytic oxygen-atom transfer to alkynes.Angew Chem Int Ed Engl. 2011 Aug 1;50(32):7226-36. doi: 10.1002/anie.201100148. Epub 2011 Jul 1. Angew Chem Int Ed Engl. 2011. PMID: 21726021 Review.
-
Manganese Alkyl Carbonyl Complexes: From Iconic Stoichiometric Textbook Reactions to Catalytic Applications.Acc Chem Res. 2022 Sep 20;55(18):2740-2751. doi: 10.1021/acs.accounts.2c00470. Epub 2022 Sep 8. Acc Chem Res. 2022. PMID: 36074912 Free PMC article. Review.
Cited by
-
PIII/PV═O-Catalyzed Intermolecular N-N Bond Formation: Cross-Selective Reductive Coupling of Nitroarenes and Anilines.J Am Chem Soc. 2021 Sep 15;143(36):14464-14469. doi: 10.1021/jacs.1c07272. Epub 2021 Sep 2. J Am Chem Soc. 2021. PMID: 34473484 Free PMC article.
-
Ti-catalyzed ring-opening oxidative amination of methylenecyclopropanes with diazenes.Chem Sci. 2020 Jun 23;11(27):7204-7209. doi: 10.1039/d0sc01998d. Chem Sci. 2020. PMID: 34123005 Free PMC article.
-
Cp2Ti(II) Mediated Rearrangement of Cyclopropyl Imines.Organometallics. 2023 Jun 26;42(12):1331-1338. doi: 10.1021/acs.organomet.3c00032. Epub 2023 Mar 15. Organometallics. 2023. PMID: 37915831 Free PMC article.
-
1,2-Addition and cycloaddition reactions of niobium bis(imido) and oxo imido complexes.Chem Sci. 2020 Oct 9;11(42):11613-11632. doi: 10.1039/d0sc03489d. Chem Sci. 2020. PMID: 34094408 Free PMC article.
-
Recent highlights in the synthesis and biological significance of pyrazole derivatives.Heliyon. 2024 Oct 9;10(20):e38894. doi: 10.1016/j.heliyon.2024.e38894. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39492900 Free PMC article. Review.
References
-
- Ansari A; Ali A; Asif M; Shamsuzzaman S Review: Biologically Active Pyrazole Derivatives. New J. Chem. 2017, 41, 16–41.
-
- Fustero S; Sánchez-Roselló M; Barrio P; Simón-Fuentes A From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034. - PubMed
-
- Knorr L Einwirkung von Acetessigester Auf Phenylhydrazin. Ber. Dtsch. Chem. Ges. 1883, 16, 2597–2599.
-
- Silva VLM; Silva AMS; Pinto DCGA; Cavaleiro JAS; Elguero J 3(5)-(2-Hydroxyphenyl)-5(3)-Styrylpyrazoles: Synthesis and Diels-Alder Transformations. Eur. J. Org. Chem. 2004, 2004, 4348–4356.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

