The diversity of ACBD proteins - From lipid binding to protein modulators and organelle tethers
- PMID: 32044385
- PMCID: PMC7057175
- DOI: 10.1016/j.bbamcr.2020.118675
The diversity of ACBD proteins - From lipid binding to protein modulators and organelle tethers
Abstract
Members of the large multigene family of acyl-CoA binding domain containing proteins (ACBDs) share a conserved motif required for binding of Coenzyme A esterified fatty acids of various chain length. These proteins are present in the three kingdoms of life, and despite their predicted roles in cellular lipid metabolism, knowledge about the precise functions of many ACBD proteins remains scarce. Interestingly, several ACBD proteins are now suggested to function at organelle contact sites, and are recognized as host interaction proteins for different pathogens including viruses and bacteria. Here, we present a thorough phylogenetic analysis of the ACBD family and discuss their structure and evolution. We summarize recent findings on the various functions of animal and fungal ACBDs with particular focus on peroxisomes, the role of ACBD proteins at organelle membranes, and their increasing recognition as targets for pathogens.
Keywords: Acyl-CoA binding domain containing protein; FFAT motif; Lipid metabolism; Membrane contact sites; Pathogen host interaction; Peroxisomes.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

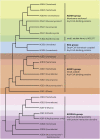



References
-
- Neess D., Bek S., Engelsby H., Gallego S.F., Færgeman N.J. Long-chain acyl-CoA esters in metabolism and signaling: role of acyl-CoA binding proteins. Prog. Lipid Res. 2015;59:1–25. - PubMed
-
- Lung S.C., Chye M.L. Deciphering the roles of acyl-CoA-binding proteins in plant cells. Protoplasma. 2016;253:1177–1195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/T019409/1/MRC_/Medical Research Council/United Kingdom
- BB/N01541X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/P003818/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/T002255/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- CIC 08135/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

