Turning liabilities into opportunities: Off-target based drug repurposing in cancer
- PMID: 32044472
- PMCID: PMC7415607
- DOI: 10.1016/j.semcancer.2020.02.003
Turning liabilities into opportunities: Off-target based drug repurposing in cancer
Abstract
Targeted drugs and precision medicine have transformed the landscape of cancer therapy and significantly improved patient outcomes in many cases. However, as therapies are becoming more and more tailored to smaller patient populations and acquired resistance is limiting the duration of clinical responses, there is an ever increasing demand for new drugs, which is not easily met considering steadily rising drug attrition rates and development costs. Considering these challenges drug repurposing is an attractive complementary approach to traditional drug discovery that can satisfy some of these needs. This is facilitated by the fact that most targeted drugs, despite their implicit connotation, are not singularly specific, but rather display a wide spectrum of target selectivity. Importantly, some of the unintended drug "off-targets" are known anticancer targets in their own right. Others are becoming recognized as such in the process of elucidating off-target mechanisms that in fact are responsible for a drug's anticancer activity, thereby revealing potentially new cancer vulnerabilities. Harnessing such beneficial off-target effects can therefore lead to novel and promising precision medicine approaches. Here, we will discuss experimental and computational methods that are employed to specifically develop single target and network-based off-target repurposing strategies, for instance with drug combinations or polypharmacology drugs. By illustrating concrete examples that have led to clinical translation we will furthermore examine the various scientific and non-scientific factors that cumulatively determine the success of these efforts and thus can inform the future development of new and potentially lifesaving off-target based drug repurposing strategies for cancers that constitute important unmet medical needs.
Keywords: Drug repurposing; Off-target; Polypharmacology; Precision medicine; Targeted cancer therapy.
Copyright © 2020. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Interest
The authors declare no competing interests.
Figures
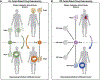
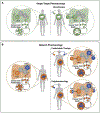
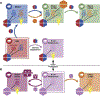
References
-
- Chin L, Andersen JN, and Futreal PA (2011). Cancer genomics: from discovery science to personalized medicine. Nat Med 17, 297–303. - PubMed
-
- Ginsburg GS, and Willard HF (2009). Genomic and personalized medicine: foundations and applications. Transl Res 154, 277–287. - PubMed
-
- Hamburg MA, and Collins FS (2010). The path to personalized medicine. N Engl J Med 363, 301–304. - PubMed
-
- Baselga J, Norton L, Albanell J, Kim YM, and Mendelsohn J (1998). Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 58, 2825–2831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

