From Shape to Function: The Next Step in Bioprinting
- PMID: 32045053
- PMCID: PMC7116209
- DOI: 10.1002/adma.201906423
From Shape to Function: The Next Step in Bioprinting
Abstract
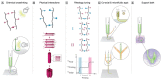
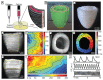
In 2013, the "biofabrication window" was introduced to reflect the processing challenge for the fields of biofabrication and bioprinting. At that time, the lack of printable materials that could serve as cell-laden bioinks, as well as the limitations of printing and assembly methods, presented a major constraint. However, recent developments have now resulted in the availability of a plethora of bioinks, new printing approaches, and the technological advancement of established techniques. Nevertheless, it remains largely unknown which materials and technical parameters are essential for the fabrication of intrinsically hierarchical cell-material constructs that truly mimic biologically functional tissue. In order to achieve this, it is urged that the field now shift its focus from materials and technologies toward the biological development of the resulting constructs. Therefore, herein, the recent material and technological advances since the introduction of the biofabrication window are briefly summarized, i.e., approaches how to generate shape, to then focus the discussion on how to acquire the biological function within this context. In particular, a vision of how biological function can evolve from the possibility to determine shape is outlined.
Keywords: biofabrication; bioinks; biological function; regenerative medicine; tissue hierarchy.
© 2020 The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Lincoln J, Lange AW, Yutzey KE. Dev Biol. 2006;294:292. - PubMed
-
- Rose JC, De Laporte L. Adv Healthcare Mater. 2018;7 1701067.
-
- Moroni L, Boland T, Burdick JA, De Maria C, Derby B, Forgacs G, Groll J, Li Q, Malda J, Mironov VA, Mota C, Nakamura M, Shu W, Takeuchi S, Woodfield TBF, Xu T, Yoo JJ, Vozzi G. Trends Biotechnol. 2018;36:384. - PubMed
-
- Groll J, Boland T, Blunk T, Burdick JA, Cho DWW, Dalton PD, Derby B, Forgacs G, Li Q, Mironov VA, Moroni L, Nakamura M, Shu W, Takeuchi S, Vozzi G, Woodfield TBFF, Xu T, Yoo JJ, Malda J. Biofabrication. 2016;8 013001. - PubMed
-
- Ozbolat IT, Hospodiuk M. Biomaterials. 2016;76:321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

