Spectroscopic Investigation of DCCH and FTSC as a potential pair for Förster Resonance Energy Transfer in different solvents
- PMID: 32045426
- PMCID: PMC7012416
- DOI: 10.1371/journal.pone.0228543
Spectroscopic Investigation of DCCH and FTSC as a potential pair for Förster Resonance Energy Transfer in different solvents
Abstract
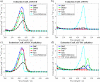
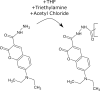
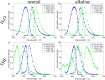
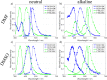
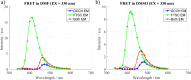
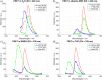
Two molecules, 7-(diethylamino)coumarin-3-carbohydrazide (DCCH) and fluorescein-5-thiosemicarbazide (FTSC) were investigated in different solvents, under varying pH conditions regarding their spectroscopic properties for the usage as a Förster Resonance Energy Transfer (FRET) pair to study the molecular interaction between cellulosic surfaces. All the relevant spectroscopic properties to determine the Förster distance were measured and the performance as a FRET system was checked. From the results, it is clear that the environmental conditions need to be accurately controlled as both, but especially the FTSC dyes are sensitive to changes. For high enough concentrations positive FRET systems were observed in DMF, DMSO, H2O, THF and alkaline DMF. However due to the low quantum yield of the unmodified DCCH throughout the investigated parameter range and the strong environmental dependency of FTSC, both dyes are not preferable for being used in a FRET system for studying interaction between cellulosic surfaces.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Th Förster. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann Phys. 1948. January 1;437(1–2):55–75.
-
- Dexter DL. A Theory of Sensitized Luminescence in Solids. The Journal of Chemical Physics. 1953. May 1;21(5):836–50.
-
- Knox RS. Förster’s resonance excitation transfer theory: not just a formula. J Biomed Opt. 2012;17(1):0110031–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

