Bidentate Chiral Bis(imidazolium)-Based Halogen-Bond Donors: Synthesis and Applications in Enantioselective Recognition and Catalysis
- PMID: 32045504
- PMCID: PMC7187470
- DOI: 10.1002/anie.201915931
Bidentate Chiral Bis(imidazolium)-Based Halogen-Bond Donors: Synthesis and Applications in Enantioselective Recognition and Catalysis
Abstract
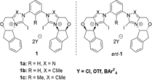
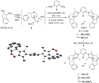
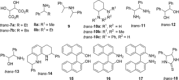
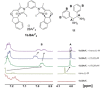
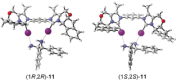
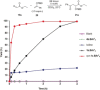
Even though halogen bonding-the noncovalent interaction between electrophilic halogen substituents and Lewis bases-has now been established in molecular recognition and catalysis, its use in enantioselective processes is still very rarely explored. Herein, we present the synthesis of chiral bidentate halogen-bond donors based on two iodoimidazolium units with rigidly attached chiral sidearms. With these Lewis acids, chiral recognition of a racemic diamine is achieved in NMR studies. DFT calculations support a 1:1 interaction of the halogen-bond donor with both enantiomers and indicate that the chiral recognition is based on a different spatial orientation of the Lewis bases in the halogen-bonded complexes. In addition, moderate enantioselectivity is achieved in a Mukaiyama aldol reaction with a preorganized variant of the chiral halogen-bond donor. This represents the first case in which asymmetric induction was realized with a pure halogen-bond donor lacking any additional active functional groups.
Keywords: chiral recognition; enantioselectivity; halogen bonding; haloimidazolium salts; organocatalysis.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Asymmetric Counteranion-Directed Halogen Bonding Catalysis.J Am Chem Soc. 2025 Mar 12;147(10):8107-8112. doi: 10.1021/jacs.4c18378. Epub 2025 Mar 3. J Am Chem Soc. 2025. PMID: 40029961 Free PMC article.
-
Stereoselective Processes Based on σ-Hole Interactions.Molecules. 2022 Jul 20;27(14):4625. doi: 10.3390/molecules27144625. Molecules. 2022. PMID: 35889497 Free PMC article. Review.
-
Neutral Chiral Tetrakis-Iodo-Triazole Halogen-Bond Donor for Chiral Recognition and Enantioselective Catalysis.Chemistry. 2021 Feb 1;27(7):2315-2320. doi: 10.1002/chem.202005016. Epub 2021 Jan 7. Chemistry. 2021. PMID: 33210767 Free PMC article.
-
Mukaiyama aldol reaction catalyzed by (benz)imidazolium-based halogen bond donors.Org Biomol Chem. 2021 Jan 28;19(4):770-774. doi: 10.1039/d0ob02503h. Epub 2021 Jan 12. Org Biomol Chem. 2021. PMID: 33432958
-
Liquid chromatography enantioseparations of halogenated compounds on polysaccharide-based chiral stationary phases: role of halogen substituents in molecular recognition.Chirality. 2015 Oct;27(10):667-84. doi: 10.1002/chir.22485. Epub 2015 Aug 3. Chirality. 2015. PMID: 26237113 Review.
Cited by
-
Asymmetric synthesis of β-amino cyanoesters with contiguous tetrasubstituted carbon centers by halogen-bonding catalysis with chiral halonium salt.Beilstein J Org Chem. 2025 Mar 12;21:547-555. doi: 10.3762/bjoc.21.43. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 40099300 Free PMC article.
-
Asymmetric Counteranion-Directed Halogen Bonding Catalysis.J Am Chem Soc. 2025 Mar 12;147(10):8107-8112. doi: 10.1021/jacs.4c18378. Epub 2025 Mar 3. J Am Chem Soc. 2025. PMID: 40029961 Free PMC article.
-
Enantioselective dearomatization reactions of heteroarenes by anion-binding organocatalysis.Chem Commun (Camb). 2023 Mar 16;59(23):3360-3372. doi: 10.1039/d2cc07101k. Chem Commun (Camb). 2023. PMID: 36790499 Free PMC article. Review.
-
Stereoselective Processes Based on σ-Hole Interactions.Molecules. 2022 Jul 20;27(14):4625. doi: 10.3390/molecules27144625. Molecules. 2022. PMID: 35889497 Free PMC article. Review.
-
Counteranion-mediated efficient iodine capture in a hexacationic imidazolium organic cage enabled by multiple non-covalent interactions.Nat Commun. 2023 Sep 28;14(1):6082. doi: 10.1038/s41467-023-41866-7. Nat Commun. 2023. PMID: 37770481 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

