Changes in the community structure of the symbiotic microbes of wild amphibians from the eastern edge of the Tibetan Plateau
- PMID: 32045512
- PMCID: PMC7142363
- DOI: 10.1002/mbo3.1004
Changes in the community structure of the symbiotic microbes of wild amphibians from the eastern edge of the Tibetan Plateau
Abstract
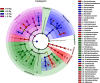
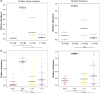
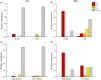
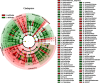
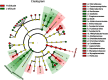
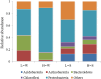
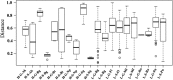
Environment has a potential effect on the animal symbiotic microbiome. Here, to study the potential relationship of the symbiotic microbiomes of wild amphibians with altitude, we collected the gut and skin samples from frogs (nine species) and the environmental samples (water and soil samples) from the Leshan Mountains (altitude: 360-410 m) and Gongga Mountains (altitude: 3340-3989 m) on the eastern edge of the Tibetan Plateau. Bufo gargarizans (Bg) samples were collected from both the Leshan and Gongga mountain regions (Bg was the only species sampled on both mountains). The DNA extracted from each sample was performed high-throughput sequencing (MiSeq) of bacterial 16S rRNA gene amplicons. High relative abundance of Caulobacteraceae and Sphingomonadaceae was found in skin samples from both Bg and the other high-altitude amphibians (nine species combined). High relative abundance of Coxiellaceae and Mycoplasmataceae was found in gut samples from both Bg and the other high-altitude amphibians. Furthermore, the alpha and beta diversities of skin and gut samples from Bg and the other amphibian species (nine species combined) were similar. In terms of the symbiotic microbial community, the low-altitude samples were less diverse and more similar to each other than the high-altitude samples were. We speculated that extreme high-altitude environments and host phylogeny may affect the amphibian microbiome. Despite the distinct microbial community differences between the skin and gut microbiomes, some functions were similar in the Bg and combined high-altitude samples. The Bg and high-altitude skin samples had higher oxidative stress tolerance and biofilm formation than the low-altitude skin samples. However, the opposite results were observed for the Bg and high-altitude gut samples. Further study is required to determine whether these characteristics favor high-altitude amphibian adaptation to extreme environments.
Keywords: Bufo gargarizans; altitude; amphibians; environmental adaptation; gut microbes; skin microbes.
© 2020 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures








Similar articles
-
Variation in the sensitivity of intestine and skin of Bufo gargarizans and Rana chensinensis tadpoles in relation to zinc exposure.Chemosphere. 2024 Sep;363:142874. doi: 10.1016/j.chemosphere.2024.142874. Epub 2024 Jul 15. Chemosphere. 2024. PMID: 39019178
-
Altitude-adaption of gut microbiota in Tibetan chicken.Poult Sci. 2022 Sep;101(9):101998. doi: 10.1016/j.psj.2022.101998. Epub 2022 Jun 10. Poult Sci. 2022. PMID: 35841636 Free PMC article.
-
The amphibian skin-associated microbiome across species, space and life history stages.Mol Ecol. 2014 Mar;23(6):1238-1250. doi: 10.1111/mec.12510. Epub 2013 Oct 31. Mol Ecol. 2014. PMID: 24171949
-
Recent progress in research on the gut microbiota and highland adaptation on the Qinghai-Tibet Plateau.J Evol Biol. 2021 Oct;34(10):1514-1530. doi: 10.1111/jeb.13924. Epub 2021 Sep 8. J Evol Biol. 2021. PMID: 34473899 Review.
-
Research Status and Prospect of Amphibian Symbiotic Microbiota.Animals (Basel). 2025 Mar 25;15(7):934. doi: 10.3390/ani15070934. Animals (Basel). 2025. PMID: 40218328 Free PMC article. Review.
Cited by
-
Hypoxia: The "Invisible Pusher" of Gut Microbiota.Front Microbiol. 2021 Jul 22;12:690600. doi: 10.3389/fmicb.2021.690600. eCollection 2021. Front Microbiol. 2021. PMID: 34367091 Free PMC article. Review.
-
The Gut Microbiome of the Asiatic Toad (Bufo gargarizans) Reflects Environmental Changes and Human Activities.Ecol Evol. 2025 May 7;15(5):e71394. doi: 10.1002/ece3.71394. eCollection 2025 May. Ecol Evol. 2025. PMID: 40342698 Free PMC article.
-
Parental care contributes to vertical transmission of microbes in a skin-feeding and direct-developing caecilian.Anim Microbiome. 2023 May 15;5(1):28. doi: 10.1186/s42523-023-00243-x. Anim Microbiome. 2023. PMID: 37189209 Free PMC article.
-
The Behavior of Amphibians Shapes Their Symbiotic Microbiomes.mSystems. 2020 Jul 28;5(4):e00626-20. doi: 10.1128/mSystems.00626-20. mSystems. 2020. PMID: 32723798 Free PMC article.
-
A novel framework unveiling the importance of heterogeneous selection and drift on the community structure of symbiotic microbial indicator taxa across altitudinal gradients in amphibians.Microbiol Spectr. 2025 Feb 4;13(2):e0419223. doi: 10.1128/spectrum.04192-23. Epub 2025 Jan 8. Microbiol Spectr. 2025. PMID: 39772705 Free PMC article.
References
-
- Anderson, M. J. (2010). A new method for non‐parametric multivariate analysis of variance. Austral Ecology, 26, 32–46.
-
- Caporaso, J. G. , Lauber, C. L. , Walters, W. A. , Berg‐Lyons, D. , Huntley, J. , Fierer, N. , … Knight, R. (2012). Ultra‐high‐throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal: Multidisciplinary Journal of Microbial Ecology, 6, 1621–1624. 10.1038/ismej.2012.8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

