Harnessing Ionic Interactions and Hydrogen Bonding for Nucleophilic Fluorination
- PMID: 32046021
- PMCID: PMC7037423
- DOI: 10.3390/molecules25030721
Harnessing Ionic Interactions and Hydrogen Bonding for Nucleophilic Fluorination
Abstract
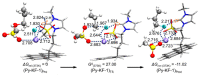
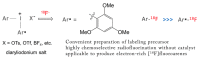
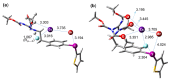
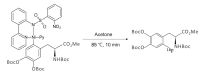
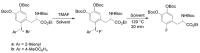
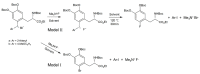
We review recent works for nucleophilic fluorination of organic compounds in which the Coulombic interactions between ionic species and/or hydrogen bonding affect the outcome of the reaction. SN2 fluorination of aliphatic compounds promoted by ionic liquids is first discussed, focusing on the mechanistic features for reaction using alkali metal fluorides. The influence of the interplay of ionic liquid cation, anion, nucleophile and counter-cation is treated in detail. The role of ionic liquid as bifunctional (both electrophilic and nucleophilic) activator is envisaged. We also review the SNAr fluorination of diaryliodonium salts from the same perspective. Nucleophilic fluorination of guanidine-containing of diaryliodonium salts, which are capable of forming hydrogen bonds with the nucleophile, is exemplified as an excellent case where ionic interactions and hydrogen bonding significantly affect the efficiency of reaction. The origin of experimental observation for the strong dependence of fluorination yields on the positions of -Boc protection is understood in terms of the location of the nucleophile with respect to the reaction center, being either close to far from it. Recent advances in the synthesis of [18F]F-dopa are also cited in relation to SNAr fluorination of diaryliodonium salts. Discussions are made with a focus on tailor-making promoters and solvent engineering based on ionic interactions and hydrogen bonding.
Keywords: hydrogen bonding; ionic interactions; nucleophilic fluorination.
Conflict of interest statement
The authors declare no conflict of interest
Figures

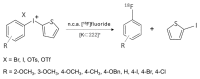
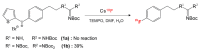

Similar articles
-
Ionic Liquids as Organocatalysts for Nucleophilic Fluorination: Concepts and Perspectives.Molecules. 2022 Sep 4;27(17):5702. doi: 10.3390/molecules27175702. Molecules. 2022. PMID: 36080470 Free PMC article. Review.
-
Facile nucleophilic fluorination reactions using tert-alcohols as a reaction medium: significantly enhanced reactivity of alkali metal fluorides and improved selectivity.J Org Chem. 2008 Feb 1;73(3):957-62. doi: 10.1021/jo7021229. Epub 2008 Jan 1. J Org Chem. 2008. PMID: 18166063
-
Bis-tert-Alcohol-Functionalized Crown-6-Calix[4]arene: An Organic Promoter for Nucleophilic Fluorination.Chemistry. 2016 Mar 18;22(13):4515-20. doi: 10.1002/chem.201504602. Epub 2016 Feb 16. Chemistry. 2016. PMID: 26880350
-
Nature of hydrogen bonding in charged hydrogen-bonded complexes and imidazolium-based ionic liquids.J Phys Chem B. 2011 Dec 15;115(49):14659-67. doi: 10.1021/jp208150b. Epub 2011 Nov 9. J Phys Chem B. 2011. PMID: 22011264
-
Hydrogen Bonding: Regulator for Nucleophilic Fluorination.Chemistry. 2017 Dec 19;23(71):17850-17861. doi: 10.1002/chem.201702664. Epub 2017 Oct 5. Chemistry. 2017. PMID: 28833711 Free PMC article. Review.
Cited by
-
Molecular Structure Modulated Trap Distribution and Carrier Migration in Fluorinated Epoxy Resin.Molecules. 2020 Jul 6;25(13):3071. doi: 10.3390/molecules25133071. Molecules. 2020. PMID: 32640527 Free PMC article.
-
Origin of Salt Effects in SN2 Fluorination Using KF Promoted by Ionic Liquids: Quantum Chemical Analysis.Molecules. 2021 Sep 22;26(19):5738. doi: 10.3390/molecules26195738. Molecules. 2021. PMID: 34641282 Free PMC article.
-
Ionic Liquids as Organocatalysts for Nucleophilic Fluorination: Concepts and Perspectives.Molecules. 2022 Sep 4;27(17):5702. doi: 10.3390/molecules27175702. Molecules. 2022. PMID: 36080470 Free PMC article. Review.
-
Multi-Interactions in Ionic Liquids for Natural Product Extraction.Molecules. 2020 Dec 28;26(1):98. doi: 10.3390/molecules26010098. Molecules. 2020. PMID: 33379318 Free PMC article. Review.
References
-
- Mascaretti O.A. Modern methods for the monofluorination of aliphatic organic compounds. Aldrichim. Acta. 1993;26:47–58.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

