The Role Played by Wnt/β-Catenin Signaling Pathway in Acute Lymphoblastic Leukemia
- PMID: 32046053
- PMCID: PMC7037748
- DOI: 10.3390/ijms21031098
The Role Played by Wnt/β-Catenin Signaling Pathway in Acute Lymphoblastic Leukemia
Abstract
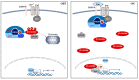
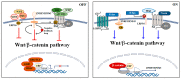
Acute lymphoblastic leukemia (ALL) is an aggressive hematologic neoplastic disorder that arises from the clonal expansion of transformed T-cell or B-cell precursors. Thanks to progress in chemotherapy protocols, ALL outcome has significantly improved. However, drug-resistance remains an unresolved issue in the treatment of ALL and toxic effects limit dose escalation of current chemotherapeutics. Therefore, the identification of novel targeted therapies to support conventional chemotherapy is required. The Wnt/β-catenin pathway is a conserved signaling axis involved in several physiological processes such as development, differentiation, and adult tissue homeostasis. As a result, deregulation of this cascade is closely related to initiation and progression of various types of cancers, including hematological malignancies. In particular, deregulation of this signaling network is involved in the transformation of healthy HSCs in leukemic stem cells (LSCs), as well as cancer cell multi-drug-resistance. This review highlights the recent findings on the role of Wnt/β-catenin in hematopoietic malignancies and provides information on the current status of Wnt/β-catenin inhibitors with respect to their therapeutic potential in the treatment of ALL.
Keywords: Wnt/β-catenin; acute lymphoblastic leukemia; hematopoietic stem cells; leukemic stem cells; signaling pathway; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Wnt Signaling in Hematological Malignancies.Prog Mol Biol Transl Sci. 2018 Jan;153:321-341. doi: 10.1016/bs.pmbts.2017.11.002. Epub 2017 Dec 29. Prog Mol Biol Transl Sci. 2018. PMID: 29389522 Free PMC article. Review.
-
Hyper-activation of WNT/β-catenin signaling pathway mediates anti-tumor effects of histone deacetylase inhibitors in acute T lymphoblastic leukemia.Leuk Lymphoma. 2012 Sep;53(9):1769-78. doi: 10.3109/10428194.2012.663085. Epub 2012 Mar 13. Leuk Lymphoma. 2012. PMID: 22303878
-
Zebrafish drug screening identifies Erlotinib as an inhibitor of Wnt/β-catenin signaling and self-renewal in T-cell acute lymphoblastic leukemia.Biomed Pharmacother. 2024 Jan;170:116013. doi: 10.1016/j.biopha.2023.116013. Epub 2023 Dec 16. Biomed Pharmacother. 2024. PMID: 38104416 Free PMC article.
-
[TIM-3 signaling hijacks the canonical Wnt/β-catenin pathway to maintain cancer stemness in human acute myeloid leukemia].Rinsho Ketsueki. 2023;64(6):547-552. doi: 10.11406/rinketsu.64.547. Rinsho Ketsueki. 2023. PMID: 37407480 Japanese.
-
The multidimensional role of the Wnt/β-catenin signaling pathway in human malignancies.J Cell Physiol. 2022 Jan;237(1):199-238. doi: 10.1002/jcp.30561. Epub 2021 Aug 25. J Cell Physiol. 2022. PMID: 34431086 Review.
Cited by
-
Chidamide and venetoclax synergistically regulate the Wnt/β-catenin pathway by MYCN/DKK3 in B-ALL.Ann Hematol. 2025 Jan;104(1):489-501. doi: 10.1007/s00277-024-06110-2. Epub 2024 Nov 28. Ann Hematol. 2025. PMID: 39607486 Free PMC article.
-
The dynamic interaction of pediatric ALL cells and MSCs: influencing leukemic cell survival and modulating MSC β-catenin expression.Histochem Cell Biol. 2025 Jan 21;163(1):26. doi: 10.1007/s00418-025-02353-w. Histochem Cell Biol. 2025. PMID: 39836255 Free PMC article.
-
Epigenetically dysregulated NOTCH-Delta-HES signaling cascade can serve as a subtype classifier for acute lymphoblastic leukemia.Ann Hematol. 2024 Feb;103(2):511-523. doi: 10.1007/s00277-023-05515-9. Epub 2023 Nov 3. Ann Hematol. 2024. PMID: 37922005
-
B-ALL Complexity: Is Targeted Therapy Still A Valuable Approach for Pediatric Patients?Cancers (Basel). 2020 Nov 24;12(12):3498. doi: 10.3390/cancers12123498. Cancers (Basel). 2020. PMID: 33255367 Free PMC article. Review.
-
Resveratrol and Its Role in the Management of B-Cell Malignancies-A Recent Update.Biomedicines. 2023 Jan 15;11(1):221. doi: 10.3390/biomedicines11010221. Biomedicines. 2023. PMID: 36672729 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

