Ginsenoside Rg3 Induces Browning of 3T3-L1 Adipocytes by Activating AMPK Signaling
- PMID: 32046061
- PMCID: PMC7071202
- DOI: 10.3390/nu12020427
Ginsenoside Rg3 Induces Browning of 3T3-L1 Adipocytes by Activating AMPK Signaling
Abstract
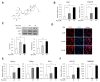
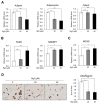
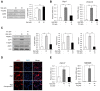
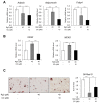
Ginsenoside Rg3, one of the major components in Panax ginseng, has been reported to possess several therapeutic effects including anti-obesity properties. However, its effect on the browning of mature white adipocytes as well as the underlying mechanism remains poorly understood. In this study, we suggested a novel role of Rg3 in the browning of mature 3T3-L1 adipocytes by upregulating browning-related gene expression. The browning effects of Rg3 on differentiated 3T3-L1 adipocytes were evaluated by analyzing browning-related markers using quantitative PCR, immunoblotting, and immunostaining. In addition, the size and sum area of lipid droplets in differentiated 3T3-L1 adipocytes were measured using Oil-Red-O staining. In mature 3T3-L1 adipocytes, Rg3 dose-dependently induced the expression of browning-related genes such as Ucp1, Prdm16, Pgc1α, Cidea, and Dio2. Moreover, Rg3 induced the expression of beige fat-specific genes (CD137 and TMEM26) and lipid metabolism-associated genes (FASN, SREBP1, and MCAD), which indicated the activation of lipid metabolism by Rg3. We also demonstrated that activation of 5' adenosine monophosphate-activated protein kinase (AMPK) is required for Rg3-mediated up-regulation of browning gene expression. Moreover, Rg3 inhibited the accumulation of lipid droplets and reduced the droplet size in mature 3T3-L1 adipocytes. Taken together, this study identifies a novel role of Rg3 in browning of white adipocytes, as well as suggesting a potential mechanism of an anti-obesity effect of Panax ginseng.
Keywords: AMPK; Rg3; anti-obesity; beige adipocytes; browning effect; ginsenoside.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Black Ginseng and Ginsenoside Rb1 Promote Browning by Inducing UCP1 Expression in 3T3-L1 and Primary White Adipocytes.Nutrients. 2019 Nov 12;11(11):2747. doi: 10.3390/nu11112747. Nutrients. 2019. PMID: 31726767 Free PMC article.
-
Naringin promotes fat browning mediated by UCP1 activation via the AMPK signaling pathway in 3T3-L1 adipocytes.Arch Pharm Res. 2023 Mar;46(3):192-205. doi: 10.1007/s12272-023-01432-7. Epub 2023 Feb 25. Arch Pharm Res. 2023. PMID: 36840853
-
Ginsenoside Rb1 promotes browning through regulation of PPARγ in 3T3-L1 adipocytes.Biochem Biophys Res Commun. 2015 Oct 23;466(3):530-5. doi: 10.1016/j.bbrc.2015.09.064. Epub 2015 Sep 14. Biochem Biophys Res Commun. 2015. PMID: 26381176
-
Anti-Angiogenic Properties of Ginsenoside Rg3.Molecules. 2020 Oct 23;25(21):4905. doi: 10.3390/molecules25214905. Molecules. 2020. PMID: 33113992 Free PMC article. Review.
-
Molecular mechanisms behind the inhibitory effects of ginsenoside Rg3 on hepatic fibrosis: a review.Arch Toxicol. 2025 Feb;99(2):541-561. doi: 10.1007/s00204-024-03941-w. Epub 2024 Dec 27. Arch Toxicol. 2025. PMID: 39729114 Review.
Cited by
-
Effect of Panax notoginseng Saponins and Major Anti-Obesity Components on Weight Loss.Front Pharmacol. 2021 Mar 25;11:601751. doi: 10.3389/fphar.2020.601751. eCollection 2020. Front Pharmacol. 2021. PMID: 33841133 Free PMC article. Review.
-
Development of an adipocyte differentiation protocol using 3T3-L1 cells for the investigation of the browning process: identification of the PPAR-γ agonist rosiglitazone as a browning reference drug.Front Pharmacol. 2025 Apr 14;16:1546456. doi: 10.3389/fphar.2025.1546456. eCollection 2025. Front Pharmacol. 2025. PMID: 40297148 Free PMC article.
-
Naringenin, a citrus flavanone, enhances browning and brown adipogenesis: Role of peroxisome proliferator-activated receptor gamma.Front Nutr. 2022 Nov 10;9:1036655. doi: 10.3389/fnut.2022.1036655. eCollection 2022. Front Nutr. 2022. PMID: 36438760 Free PMC article.
-
Natural Extracts That Stimulate Adipocyte Browning and Their Underlying Mechanisms.Antioxidants (Basel). 2021 Feb 17;10(2):308. doi: 10.3390/antiox10020308. Antioxidants (Basel). 2021. PMID: 33671335 Free PMC article. Review.
-
Protective Effects of Red Ginseng Against Tacrine-Induced Hepatotoxicity: An Integrated Approach with Network Pharmacology and Experimental Validation.Drug Des Devel Ther. 2024 Feb 23;18:549-566. doi: 10.2147/DDDT.S450305. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38419811 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

