WNT5a-ROR Signaling Is Essential for Alveologenesis
- PMID: 32046118
- PMCID: PMC7072327
- DOI: 10.3390/cells9020384
WNT5a-ROR Signaling Is Essential for Alveologenesis
Abstract
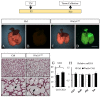
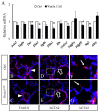
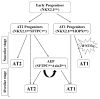
WNT5a is a mainly "non-canonical" WNT ligand whose dysregulation is observed in lung diseases such as idiopathic pulmonary fibrosis (IPF), chronic obstructive pulmonary disease (COPD) and asthma. Germline deletion of Wnt5a disrupts embryonic lung development. However, the temporal-specific function of WNT5a remains unknown. In this study, we generated a conditional loss-of-function mouse model (Wnt5aCAG) and examined the specific role of Wnt5a during the saccular and alveolar phases of lung development. The lack of Wnt5a in the saccular phase blocked distal airway expansion and attenuated differentiation of endothelial and alveolar epithelial type I (AT1) cells and myofibroblasts. Postnatal Wnt5a inactivation disrupted alveologenesis, producing a phenotype resembling human bronchopulmonary dysplasia (BPD). Mutant lungs showed hypoalveolization, but endothelial and epithelial differentiation was unaffected. The major impact of Wnt5a inactivation on alveologenesis was on myofibroblast differentiation and migration, with reduced expression of key regulatory genes. These findings were validated in vitro using isolated lung fibroblasts. Conditional inactivation of the WNT5a receptors Ror1 and Ror2 in alveolar myofibroblasts recapitulated the Wnt5aCAG phenotype, demonstrating that myofibroblast defects are the major cause of arrested alveologenesis in Wnt5aCAG lungs. Finally, we show that WNT5a is reduced in human BPD lung samples, indicating the clinical relevance and potential role for WNT5a in pathogenesis of BPD.
Keywords: ROR; WNT5a; alveologenesis; lung; migration; secondary crest myofibroblast.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures






References
-
- Frank D.B., Penkala I.J., Zepp J.A., Sivakumar A., Linares-Saldana R., Zacharias W.J., Stolz K.G., Pankin J., Lu M., Wang Q., et al. Early lineage specification defines alveolar epithelial ontogeny in the murine lung. Proc. Natl. Acad. Sci. 2019;116:4362–4371. doi: 10.1073/pnas.1813952116. - DOI - PMC - PubMed
-
- Boström H., Willetts K., Pekny M., Levéen P., Lindahl P., Hedstrand H., Pekna M., Hellström M., Gebre-Medhin S., Schalling M., et al. PDGF-A Signaling Is a Critical Event in Lung Alveolar Myofibroblast Development and Alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/S0092-8674(00)81270-2. - DOI - PubMed
-
- Lindahl P., Karlsson L., Hellström M., Gebre-Medhin S., Willetts K., Heath J.K., Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–3953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

