Cording Mycobacterium tuberculosis Bacilli Have a Key Role in the Progression towards Active Tuberculosis, Which is Stopped by Previous Immune Response
- PMID: 32046344
- PMCID: PMC7074780
- DOI: 10.3390/microorganisms8020228
Cording Mycobacterium tuberculosis Bacilli Have a Key Role in the Progression towards Active Tuberculosis, Which is Stopped by Previous Immune Response
Abstract
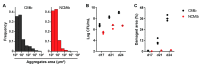
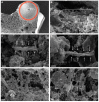
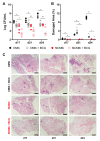
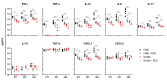
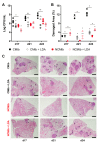
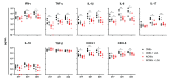
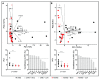
Cording was the first virulence factor identified in Mycobacterium tuberculosis (Mtb). We aimed to ascertain its role in the induction of active tuberculosis (TB) in the mouse strain C3HeB/FeJ by testing the immunopathogenic capacity of the H37Rv strain. We have obtained two batches of the same strain by stopping their growth in Proskauer Beck liquid medium once the mid-log phase was reached, in the noncording Mtb (NCMtb) batch, and two days later in the cording Mtb (CMtb) batch, when cording could be detected by microscopic analysis. Mice were challenged with each batch intravenously and followed-up for 24 days. CMtb caused a significant increase in the bacillary load at an early stage post-challenge (day 17), when a granulomatous response started, generating exudative lesions characterized by neutrophilic infiltration, which promoted extracellular bacillary growth together with cording formation, as shown for the first time in vivo. In contrast, NCMtb experienced slight or no bacillary growth and lesions could barely be detected. Previous Bacillus Calmette-Guérin (BCG) vaccination or low dose aerosol (LDA) Mtb infection were able to delay the progression towards active TB after CMtb challenge. While BCG vaccination also reduced bacillary load when NCMtb was challenged, LDA did not, and its proliferative lesions experienced neutrophil infiltration. Analysis of lung cytokine and chemokine profiles points to their capacity to block the production of CXCL-1 and further amplification of IL-1β, IL-17 and neutrophilic extracellular trap formation, all of which are essential for TB progression. These data highlight the key role of cording formation in the induction of active TB.
Keywords: BCG; C3HeB/FeJ mice; CXCL-1; Mycobacterium tuberculosis; cording; neutrophilic extracellular traps; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- WHO Global Tuberculosis Report 2018. WHO; Geneva, Switzerland: 2018.
-
- Cardona P.-J., Català M., Arch M., Arias L., Alonso S., Cardona P., López D., Vilaplana C., Prats C. Can systems immunology lead tuberculosis eradication? Curr. Opin. Syst. Biol. 2018;12:53–60. doi: 10.1016/j.coisb.2018.10.004. - DOI
-
- Menzies N.A., Wolf E., Connors D., Bellerose M., Sbarra A.N., Cohen T., Hill A.N., Yaesoubi R., Galer K., White P.J., et al. Progression from latent infection to active disease in dynamic tuberculosis transmission models: A systematic review of the validity of modelling assumptions. Lancet Infect. Dis. 2018;18:e228–e238. doi: 10.1016/S1473-3099(18)30134-8. - DOI - PMC - PubMed
Grants and funding
- TBVAC2020 nº 643381/European Commission Horizon 2020
- LCF/PR/GN16/10290002/La Caixa Foundation (ID 100010434)
- 2017 SGR500/Catalan Agency for Management of University and Research Grants (AGAUR)
- CPII18/00031/Plan Nacional I + D + I co-financed by ISCIII-Subdirección General de Evaluación and Fondo-EU de Desarrollo Regional (FEDER)
- IFI14/00015/Plan Nacional I + D + I co-financed by ISCIII-Subdirección General de Evaluación and Fondo-EU de Desarrollo Regional (FEDER)
LinkOut - more resources
Full Text Sources

