NAV3, a Tumor Suppressor Gene, Is Decreased in Uterine Leiomyoma Tissue and Cells
- PMID: 32046415
- PMCID: PMC7539815
- DOI: 10.1007/s43032-019-00096-3
NAV3, a Tumor Suppressor Gene, Is Decreased in Uterine Leiomyoma Tissue and Cells
Abstract
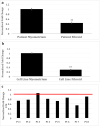
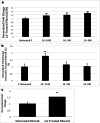
NAV 3 is a tumor suppressor of unknown function in leiomyomas. The objective of this study is to assess NAV3 expression and its potential role in human uterine leiomyomas. NAV3 protein expression was examined in patient leiomyoma and patient-matched myometrial tissue samples by Western blot and immunohistochemistry. NAV3 mRNA and protein expression was assessed in leuprolide acetate- and cetrorelix-treated cell line leiomyoma samples. RNAseq analysis of placebo-treated leiomyoma compared with myometrium demonstrated the presence of transcripts encoding for several neuronal proteins. For NAV3, RNA sequence analysis demonstrated decreased expression in leiomyoma as compared with myometrium (0.86 ± 0.03 fold). Presence of NAV3 mRNA was also decreased in leiomyoma surgical samples (0.43 fold ± 0.05, p = 0.026) compared with patient-matched myometrium. Confirmatory qRT-PCR results on immortalized leiomyoma and myometrial cell lines similarly demonstrated a decrease in expression of NAV3 in leiomyomas (0.28 ± 0.02, p = 0.00075). Immunohistochemical analysis demonstrated a significant decrease in NAV 3 protein in leiomyomas (H-score 154.7 ± 6.2) as compared with myometrium (H-score; 312.5 ± 14.7, p < 0.0001). Leuprolide acetate-treated leiomyoma cells demonstrated an increase in NAV 3 mRNA expression (1.53 ± 0.13, p < 0.0001). Similarly, Western blot analysis on leuprolide-treated leiomyoma cells showed a non-significant increase in NAV 3 protein expression (1.26 ± 0.09, p = 0.063). NAV 3, a tumor suppressor in numerous cancers, is decreased in leiomyoma cells and tissue compared with myometrium, and increased by GnRH analog treatment, suggesting that NAV3 may mediate steroid hormone-independent leiomyoma regulation by GnRH analogs.
Keywords: Cetrorelix; GnRH; Leiomyoma; Leuprolide acetate; Myometrium; NAV3; Tumor suppressor gene.
Figures

Similar articles
-
Gonadotropin-releasing hormone (GnRH) agonist leuprolide acetate and GnRH antagonist cetrorelix acetate directly inhibit leiomyoma extracellular matrix production.Fertil Steril. 2012 Nov;98(5):1299-307. doi: 10.1016/j.fertnstert.2012.07.1123. Epub 2012 Aug 14. Fertil Steril. 2012. PMID: 22901846
-
Steroid hormones and hormone antagonists regulate the neural marker neurotrimin in uterine leiomyoma.Fertil Steril. 2020 Jan;113(1):176-186. doi: 10.1016/j.fertnstert.2019.08.090. Fertil Steril. 2020. PMID: 32033718 Clinical Trial.
-
CCNs, fibulin-1C and S100A4 expression in leiomyoma and myometrium: inverse association with TGF-beta and regulation by TGF-beta in leiomyoma and myometrial smooth muscle cells.Mol Hum Reprod. 2006 Apr;12(4):245-56. doi: 10.1093/molehr/gal015. Epub 2006 Mar 29. Mol Hum Reprod. 2006. Retraction in: Mol Hum Reprod. 2014 Dec;20(12):1258. doi: 10.1093/molehr/gau100. PMID: 16571622 Retracted.
-
Isolation and characterization of heparin-binding growth factors in human leiomyomas and normal myometrium.Biol Reprod. 1995 Sep;53(3):636-46. doi: 10.1095/biolreprod53.3.636. Biol Reprod. 1995. PMID: 7578688
-
Differential effects of tumor necrosis factor-α on matrix metalloproteinase-2 expression in human myometrial and uterine leiomyoma smooth muscle cells.Hum Reprod. 2015 Jan;30(1):61-70. doi: 10.1093/humrep/deu300. Epub 2014 Nov 14. Hum Reprod. 2015. PMID: 25398968
Cited by
-
GWAS meta-analysis identifies five susceptibility loci for endometrial cancer.EBioMedicine. 2025 Aug;118:105830. doi: 10.1016/j.ebiom.2025.105830. Epub 2025 Jul 8. EBioMedicine. 2025. PMID: 40633141 Free PMC article.
-
NAV3 Is a Novel Prognostic Biomarker Affecting the Immune Status of the Tumor Microenvironment in Colorectal Cancer.J Immunol Res. 2022 Jun 30;2022:8337048. doi: 10.1155/2022/8337048. eCollection 2022. J Immunol Res. 2022. PMID: 35812247 Free PMC article.
-
Tumour Suppressor Neuron Navigator 3 and Matrix Metalloproteinase 14 are Co-expressed in Most Melanomas but Downregulated in Thick Tumours.Acta Derm Venereol. 2023 Mar 8;103:adv00883. doi: 10.2340/actadv.v103.298. Acta Derm Venereol. 2023. PMID: 36883877 Free PMC article.
-
Whole-genome resequencing to investigate the genetic diversity and mechanisms of plateau adaptation in Tibetan sheep.J Anim Sci Biotechnol. 2024 Dec 6;15(1):164. doi: 10.1186/s40104-024-01125-1. J Anim Sci Biotechnol. 2024. PMID: 39639384 Free PMC article.
References
-
- Varghese BV, Koohestani F, McWilliams M, Colvin A, Gunewardena S, Kinsey WH, et al. Loss of the repressor REST in uterine fibroids promotes aberrant G protein-coupled receptor 10 expression and activates mammalian target of rapamycin pathway. Proc Natl Acad Sci. 2013;110:2187–2192. doi: 10.1073/pnas.1215759110. - DOI - PMC - PubMed
-
- Coy JF, Wiemann S, Bechmann I, Bächner D, Nitsch R, Kretz O, Christiansen H, Poustka A. Pore membrane and/or filament interacting like protein 1 (POMFIL1) is predominantly expressed in the nervous system and encodes different protein isoforms. Gene. 2002;290:73–94. doi: 10.1016/S0378-1119(02)00567-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

