Introduction of Somatic Mutation in MED12 Induces Wnt4/β-Catenin and Disrupts Autophagy in Human Uterine Myometrial Cell
- PMID: 32046450
- PMCID: PMC7539814
- DOI: 10.1007/s43032-019-00084-7
Introduction of Somatic Mutation in MED12 Induces Wnt4/β-Catenin and Disrupts Autophagy in Human Uterine Myometrial Cell
Abstract
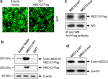
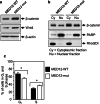
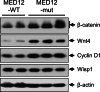
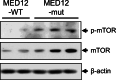
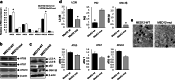
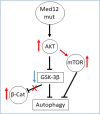
Uterine fibroids (UFs) or leiomyoma are frequently associated with somatic mutations in the mediator complex subunit 12 (MED12) gene; however, the function of these mutations in human UF biology is yet to be determined. Herein, we determined the functional role of the most common MED12 somatic mutation in the modulation of oncogenic Wnt4/β-catenin and mammalian target of rapamycin (mTOR) signaling pathways. Using an immortalized human uterine myometrial smooth muscle cell line (UtSM), we constitutively overexpressed either MED12-Wild Type or the most common MED12 somatic mutation (c.131G>A), and the effects of this MED12 mutation were compared between these cell lines. This immortalized cell line was used as a model because it expresses wild type MED12 protein and do not possess MED12 somatic mutations. By comparing the effect between MED12-WT and MED12-mutant (mut) stable cell populations, we observed increased levels of protein expression of Wnt4 and β-catenin in MED12-mut cells as compared with MED12-WT cells. MED12-mut cells also expressed increased levels of mTOR protein and oncogenic cyclin D1 which are hallmarks of cell growth and tumorigenicity. This somatic mutation in MED12 showed an effect on cell-cycle progression by induction of S-phase cells. MED12-mut cells also showed inhibition of autophagy as compared with MED12-WT cells. Together, these findings indicate that the MED12 somatic mutation has the potentials for myometrial cell transformation by dysregulating oncogenic Wnt4/β-catenin and its downstream mTOR signaling which might be associated with autophagy abrogation, cell proliferation, and tumorigenicity.
Keywords: Leiomyoma; MED12; Somatic mutations; Uterine fibroid.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Vitamin D3 Inhibits Wnt/β-Catenin and mTOR Signaling Pathways in Human Uterine Fibroid Cells.J Clin Endocrinol Metab. 2016 Apr;101(4):1542-51. doi: 10.1210/jc.2015-3555. Epub 2016 Jan 28. J Clin Endocrinol Metab. 2016. PMID: 26820714 Free PMC article.
-
Silencing Med12 Gene Reduces Proliferation of Human Leiomyoma Cells Mediated via Wnt/β-Catenin Signaling Pathway.Endocrinology. 2017 Mar 1;158(3):592-603. doi: 10.1210/en.2016-1097. Endocrinology. 2017. PMID: 27967206 Free PMC article.
-
Vitamin D as an effective treatment in human uterine leiomyomas independent of mediator complex subunit 12 mutation.Fertil Steril. 2021 Feb;115(2):512-521. doi: 10.1016/j.fertnstert.2020.07.049. Epub 2020 Oct 7. Fertil Steril. 2021. PMID: 33036796
-
Epidemiological and genetic clues for molecular mechanisms involved in uterine leiomyoma development and growth.Hum Reprod Update. 2015 Sep-Oct;21(5):593-615. doi: 10.1093/humupd/dmv030. Epub 2015 Jul 3. Hum Reprod Update. 2015. PMID: 26141720 Free PMC article. Review.
-
MED12 and uterine smooth muscle oncogenesis: State of the art and perspectives.Eur J Cancer. 2015 Aug;51(12):1603-10. doi: 10.1016/j.ejca.2015.04.023. Epub 2015 May 30. Eur J Cancer. 2015. PMID: 26037152 Review.
Cited by
-
Simvastatin inhibits stem cell proliferation in human leiomyoma via TGF-β3 and Wnt/β-Catenin pathways.J Cell Mol Med. 2022 Mar;26(5):1684-1698. doi: 10.1111/jcmm.17211. Epub 2022 Feb 4. J Cell Mol Med. 2022. PMID: 35118811 Free PMC article.
-
The Evolving Role of Natural Compounds in the Medical Treatment of Uterine Fibroids.J Clin Med. 2020 May 14;9(5):1479. doi: 10.3390/jcm9051479. J Clin Med. 2020. PMID: 32423112 Free PMC article. Review.
-
Gene variants polymorphisms and uterine leiomyoma: an updated review.Front Genet. 2024 Mar 20;15:1330807. doi: 10.3389/fgene.2024.1330807. eCollection 2024. Front Genet. 2024. PMID: 38572418 Free PMC article. Review.
-
Genomic Characterization and Clinical Outcomes of Patients with Peritoneal Metastases from the AACR GENIE Biopharma Collaborative Colorectal Cancer Registry.Cancer Res Commun. 2024 Feb 20;4(2):475-486. doi: 10.1158/2767-9764.CRC-23-0409. Cancer Res Commun. 2024. PMID: 38329392 Free PMC article.
-
The Effect of Race/Ethnicity and MED12 Mutation on the Expression of Long Non-Coding RNAs in Uterine Leiomyoma and Myometrium.Int J Mol Sci. 2024 Jan 21;25(2):1307. doi: 10.3390/ijms25021307. Int J Mol Sci. 2024. PMID: 38279317 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

