Antiallodynic and anti-inflammatory effects of intrathecal R-PIA in a rat model of vincristine-induced peripheral neuropathy
- PMID: 32046474
- PMCID: PMC7533172
- DOI: 10.4097/kja.19481
Antiallodynic and anti-inflammatory effects of intrathecal R-PIA in a rat model of vincristine-induced peripheral neuropathy
Abstract
Background: Studies investigating the correlation between spinal adenosine A1 receptors and vincristine-induced peripheral neuropathy (VIPN) are limited. This study explored the role of intrathecal N6-(2-phenylisopropyl)-adenosine R-(-)isomer (R-PIA) in the rat model of VIPN.
Methods: Vincristine (100 μg/kg) was intraperitoneally administered for 10 days (two 5-day cycles with a 2-day pause) and VIPN was induced in rats. Pain was assessed by evaluating mechanical hyperalgesia, mechanical dynamic allodynia, thermal hyperalgesia, cold allodynia, and mechanical static allodynia. Biochemically, tumor necrosis factor-alpha (TNF-α) level and myeloperoxidase (MPO) activity were measured in the tissue from beneath the sciatic nerve.
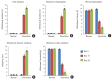
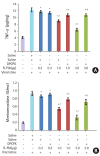
Results: Vincristine administration resulted in the development of cold allodynia, mechanical hyperalgesia, thermal hyperalgesia, mechanical dynamic allodynia, and mechanical static allodynia. Intrathecally administered R-PIA (1.0 and 3.0 μg/10 μl) reversed vincristine-induced neuropathic pain (cold and mechanical static allodynia). The attenuating effect peaked 15 min after intrathecal administration of R-PIA after which it decreased until 180 min. However, pretreatment with 1,3-dipropyl-8-cyclopentylxanthine (DPCPX, 10 μg/10 μl) 15 min before intrathecal R-PIA administration significantly attenuated the antiallodynic effect of R-PIA. This antiallodynic effect of intrathecal R-PIA may be mediated through adenosine A1 receptors in the spinal cord. Intrathecally administered R-PIA also attenuated vincristine-induced increases in TNF-α level and MPO activity. However, pretreatment with intrathecal DPCPX significantly reversed this attenuation.
Conclusions: These results suggest that intrathecally administered R-PIA attenuates cold and mechanical static allodynia in a rat model of VIPN, partially due to its anti-inflammatory actions.
Keywords: Adenosine; DPCPX; Neuropathy; R-PIA; Receptor; Vincristine..
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6:657–66. - PubMed
-
- Silva A, Wang Q, Wang M, Ravula SK, Glass JD. Evidence for direct axonal toxicity in vincristine neuropathy. J Peripher Nerv Syst. 2006;11:211–6. - PubMed
-
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

