MicroRNA-497-5p Functions as a Modulator of Apoptosis by Regulating Metadherin in Ovarian Cancer
- PMID: 32046519
- PMCID: PMC7444230
- DOI: 10.1177/0963689719897061
MicroRNA-497-5p Functions as a Modulator of Apoptosis by Regulating Metadherin in Ovarian Cancer
Abstract
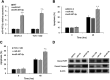
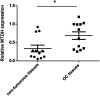
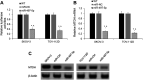
Ovarian cancer (OC) has a high mortality rate among women worldwide. However, even with the advances in detection and therapeutics, the number of cases is increasing worldwide. Increasingly, microRNAs (miRNAs), including miR-497-5p, have been implicated in the progression of many cancers, but the role of miR-497-5p in OC remains unknown. The purpose of this study was to investigate the underlying molecular mechanism of miR-497-5p in OC. Herein, we find that miR-497-5p is down-regulated in OC tissues, and overexpression of miR-497-5p enhances apoptosis in OC cells. The increased apoptosis was correlated with enhanced expression of apoptosis-related proteins. MiR-497-5p directly bound the 3'-untranslated region of metadherin (MTDH), leading to the reduction of MTDH in mRNA and protein levels. Moreover, MTDH knockout promoted the apoptosis of OC cells. Taken together, we conclude that miR-497-5p contributes to cell apoptosis in OC by regulating MTDH.
Keywords: MTDH; apoptosis; miR-497-5p; ovarian cancer.
Conflict of interest statement
Figures


Similar articles
-
F-Box and Leucine-Rich Repeat Protein 20 (FBXL20), Negatively Regulated by microRNA (miR)-195-5p, Accelerates the Malignant Progression of Ovarian Cancer.Mol Biotechnol. 2021 Dec;63(12):1235-1243. doi: 10.1007/s12033-021-00375-y. Epub 2021 Aug 2. Mol Biotechnol. 2021. PMID: 34338995
-
MicroRNA-204-5p Inhibits Ovarian Cancer Cell Proliferation by Down-Regulating USP47.Cell Transplant. 2019 Dec;28(1_suppl):51S-58S. doi: 10.1177/0963689719877372. Epub 2019 Sep 17. Cell Transplant. 2019. PMID: 31526052 Free PMC article.
-
MiR-17-5p up-regulates YES1 to modulate the cell cycle progression and apoptosis in ovarian cancer cell lines.J Cell Biochem. 2015 Jun;116(6):1050-9. doi: 10.1002/jcb.25060. J Cell Biochem. 2015. Retraction in: J Cell Biochem. 2021 Nov;122 Suppl 1:S136. doi: 10.1002/jcb.30132. PMID: 25561420 Retracted.
-
A Review of AEG-1 Oncogene Regulating MicroRNA Expression in Colon Cancer Progression.Endocr Metab Immune Disord Drug Targets. 2021;21(1):27-34. doi: 10.2174/1871530320666200618104116. Endocr Metab Immune Disord Drug Targets. 2021. PMID: 32552658 Review.
-
RNA-binding proteins in ovarian cancer: a novel avenue of their roles in diagnosis and treatment.J Transl Med. 2022 Jan 21;20(1):37. doi: 10.1186/s12967-022-03245-6. J Transl Med. 2022. PMID: 35062979 Free PMC article. Review.
Cited by
-
Integrated miRNA-mRNA Expression Profiles Revealing Key Molecules in Ovarian Cancer Based on Bioinformatics Analysis.Biomed Res Int. 2021 Oct 25;2021:6673655. doi: 10.1155/2021/6673655. eCollection 2021. Biomed Res Int. 2021. PMID: 34734085 Free PMC article.
-
Long noncoding RNA SNHG25 promotes the malignancy of endometrial cancer by sponging microRNA-497-5p and increasing FASN expression.J Ovarian Res. 2021 Nov 18;14(1):163. doi: 10.1186/s13048-021-00906-w. J Ovarian Res. 2021. PMID: 34789312 Free PMC article.
-
Circ_0005198 enhances temozolomide resistance of glioma cells through miR-198/TRIM14 axis.Aging (Albany NY). 2020 Dec 9;13(2):2198-2211. doi: 10.18632/aging.202234. Epub 2020 Dec 9. Aging (Albany NY). 2020. PMID: 33316781 Free PMC article.
-
Identification of DGUOK-AS1 as a Prognostic Factor in Breast Cancer by Bioinformatics Analysis.Front Oncol. 2020 Jul 17;10:1092. doi: 10.3389/fonc.2020.01092. eCollection 2020. Front Oncol. 2020. PMID: 32766141 Free PMC article.
-
Knockdown of circPUM1 impedes cell growth, metastasis and glycolysis of papillary thyroid cancer via enhancing MAPK1 expression by serving as the sponge of miR-21-5p.Genes Genomics. 2021 Feb;43(2):141-150. doi: 10.1007/s13258-020-01023-6. Epub 2021 Jan 22. Genes Genomics. 2021. PMID: 33481227
References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. - PubMed
-
- Deb B, Uddin A, Chakraborty S. miRNAs and ovarian cancer: an overview. J Cell Physiol. 2018;233(5):3846–3854. - PubMed
-
- Zhao H, Liu S, Wang G, Wu X, Ding Y, Guo G, Jiang J, Cui S. Expression of miR-136 is associated with the primary cisplatin resistance of human epithelial ovarian cancer. Oncol Rep. 2015;33(2):591–598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

