Mapping Subcortical Brain Alterations in 22q11.2 Deletion Syndrome: Effects of Deletion Size and Convergence With Idiopathic Neuropsychiatric Illness
- PMID: 32046535
- PMCID: PMC7419015
- DOI: 10.1176/appi.ajp.2019.19060583
Mapping Subcortical Brain Alterations in 22q11.2 Deletion Syndrome: Effects of Deletion Size and Convergence With Idiopathic Neuropsychiatric Illness
Abstract
Objective: 22q11.2 deletion syndrome (22q11DS) is among the strongest known genetic risk factors for schizophrenia. Previous studies have reported variable alterations in subcortical brain structures in 22q11DS. To better characterize subcortical alterations in 22q11DS, including modulating effects of clinical and genetic heterogeneity, the authors studied a large multicenter neuroimaging cohort from the ENIGMA 22q11.2 Deletion Syndrome Working Group.
Methods: Subcortical structures were measured using harmonized protocols for gross volume and subcortical shape morphometry in 533 individuals with 22q11DS and 330 matched healthy control subjects (age range, 6-56 years; 49% female).
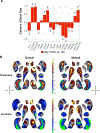
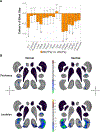
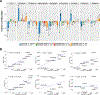
Results: Compared with the control group, the 22q11DS group showed lower intracranial volume (ICV) and thalamus, putamen, hippocampus, and amygdala volumes and greater lateral ventricle, caudate, and accumbens volumes (Cohen's d values, -0.90 to 0.93). Shape analysis revealed complex differences in the 22q11DS group across all structures. The larger A-D deletion was associated with more extensive shape alterations compared with the smaller A-B deletion. Participants with 22q11DS with psychosis showed lower ICV and hippocampus, amygdala, and thalamus volumes (Cohen's d values, -0.91 to 0.53) compared with participants with 22q11DS without psychosis. Shape analysis revealed lower thickness and surface area across subregions of these structures. Compared with subcortical findings from other neuropsychiatric disorders studied by the ENIGMA consortium, significant convergence was observed between participants with 22q11DS with psychosis and participants with schizophrenia, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder.
Conclusions: In the largest neuroimaging study of 22q11DS to date, the authors found widespread alterations to subcortical brain structures, which were affected by deletion size and psychotic illness. Findings indicate significant overlap between 22q11DS-associated psychosis, idiopathic schizophrenia, and other severe neuropsychiatric illnesses.
Keywords: 22q11.2 Deletion Syndrome; Copy Number Variant; Neuroanatomy; Neurodevelopment; Psychosis; Schizophrenia.
Conflict of interest statement
Disclosures
Declan G. Murphy has received honoraria from Roche. Paul M. Thompson and Christopher R. K. Ching have received grant support from Biogen, Inc. (Boston, USA) for work unrelated to the topic of this manuscript. All other authors have no conflicts of interest to disclose.
Figures

Comment in
-
Subcortical Signatures of Hemizygosity and Psychosis in 22q11.2 Deletion Syndrome: Finding Common Ground in Rare Genetic Variation.Am J Psychiatry. 2020 Jul 1;177(7):564-566. doi: 10.1176/appi.ajp.2020.20050598. Am J Psychiatry. 2020. PMID: 32605438 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
- P41 EB015922/EB/NIBIB NIH HHS/United States
- T32 MH073526/MH/NIMH NIH HHS/United States
- U01 MH101719/MH/NIMH NIH HHS/United States
- R01 MH085953/MH/NIMH NIH HHS/United States
- R01 MH111671/MH/NIMH NIH HHS/United States
- R01 MH116147/MH/NIMH NIH HHS/United States
- T32 AG058507/AG/NIA NIH HHS/United States
- U54 EB020403/EB/NIBIB NIH HHS/United States
- R56 AG058854/AG/NIA NIH HHS/United States
- R37 MH085953/MH/NIMH NIH HHS/United States
- R01 MH119185/MH/NIMH NIH HHS/United States
- K01 MH102609/MH/NIMH NIH HHS/United States
- R01 MH064824/MH/NIMH NIH HHS/United States
- R01 MH100900/MH/NIMH NIH HHS/United States
- MR/N026063/1/MRC_/Medical Research Council/United Kingdom
- R01 GM125757/GM/NIGMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- U01 MH101723/MH/NIMH NIH HHS/United States
- MR/L010305/1/MRC_/Medical Research Council/United Kingdom
- U01 MH087636/MH/NIMH NIH HHS/United States
- P01 HD070454/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

