Protective effects of a food-grade recombinant Lactobacillus plantarum with surface displayed AMA1 and EtMIC2 proteins of Eimeria tenella in broiler chickens
- PMID: 32046719
- PMCID: PMC7014946
- DOI: 10.1186/s12934-020-1297-4
Protective effects of a food-grade recombinant Lactobacillus plantarum with surface displayed AMA1 and EtMIC2 proteins of Eimeria tenella in broiler chickens
Abstract
Background: Avian coccidiosis posts a severe threat to poultry production. In addition to commercial attenuated vaccines, other strategies to combat coccidiosis are urgently needed. Lactobacillus plantarum has been frequently used for expression of foreign proteins as an oral vaccine delivery system using traditional erythromycin resistance gene (erm). However, antibiotic selection markers were often used during protein expression and they pose a risk of transferring antibiotic resistance genes to the environment, and significantly restricting the application in field production. Therefore, a food-grade recombinant L. plantarum vaccine candidate would dramatically improve its application potential in the poultry industry.
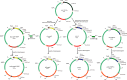
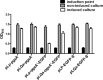
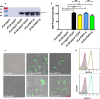
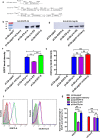
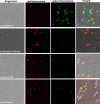
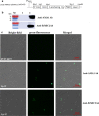
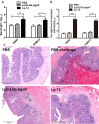
Results: In this study, we firstly replaced the erythromycin resistance gene (erm) of the pLp_1261Inv-derived expression vector with a non-antibiotic, asd-alr fusion gene, yielding a series of non-antibiotic and reliable, food grade expression vectors. In addition, we designed a dual-expression vector that displayed two foreign proteins on the surface of L. plantarum using the anchoring sequences from either a truncated poly-γ-glutamic acid synthetase A (pgsA') from Bacillus subtilis or the L. acidophilus surface layer protein (SlpA). EGFP and mCherry were used as marker proteins to evaluate the surface displayed properties of recombinant L. plantarum strains and were inspected by western blot, flow cytometry and fluorescence microscopy. To further determine its application as oral vaccine candidate, the AMA1 and EtMIC2 genes of E. tenella were anchored on the surface of L. plantarum strain. After oral immunization in chickens, the recombinant L. plantarum strain was able to induce antigen specific humoral, mucosal, and T cell-mediated immune responses, providing efficient protection against coccidiosis challenge.
Conclusions: The novel constructed food grade recombinant L. plantarum strain with double surface displayed antigens provides a potential efficient oral vaccine candidate for coccidiosis.
Keywords: Eimeria tenella; Food-grade; Lactobacillus plantarum; Surface displayed expression.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Recombinant invasive Lactobacillus plantarum expressing the Eimeria tenella fusion gene TA4 and AMA1 induces protection against coccidiosis in chickens.Vet Parasitol. 2020 Jul;283:109161. doi: 10.1016/j.vetpar.2020.109161. Epub 2020 Jun 2. Vet Parasitol. 2020. PMID: 32526607
-
A potential vaccine candidate towards chicken coccidiosis mediated by recombinant Lactobacillus plantarum with surface displayed EtMIC2 protein.Exp Parasitol. 2020 Aug;215:107901. doi: 10.1016/j.exppara.2020.107901. Epub 2020 Jun 7. Exp Parasitol. 2020. PMID: 32525007
-
Oral vaccination with a recombinant Lactobacillus plantarum expressing the Eimeria tenella rhoptry neck 2 protein elicits protective immunity in broiler chickens infected with Eimeria tenella.Parasit Vectors. 2024 Jun 28;17(1):277. doi: 10.1186/s13071-024-06355-w. Parasit Vectors. 2024. PMID: 38943202 Free PMC article.
-
Progress in recombinant vaccine development against coccidiosis. A review and prospects into the next millennium.Int J Parasitol. 1998 Jul;28(7):1121-30. doi: 10.1016/s0020-7519(98)00080-0. Int J Parasitol. 1998. PMID: 9724883 Review.
-
Vaccines against chicken coccidiosis with particular reference to previous decade: progress, challenges, and opportunities.Parasitol Res. 2022 Oct;121(10):2749-2763. doi: 10.1007/s00436-022-07612-6. Epub 2022 Aug 4. Parasitol Res. 2022. PMID: 35925452 Free PMC article. Review.
Cited by
-
Immune-Stimulating Potential of Lacticaseibacillus rhamnosus LM1019 in RAW 264.7 Cells and Immunosuppressed Mice Induced by Cyclophosphamide.Microorganisms. 2023 Sep 13;11(9):2312. doi: 10.3390/microorganisms11092312. Microorganisms. 2023. PMID: 37764156 Free PMC article.
-
Lactiplantibacillus plantarum: a new example of inclusion body producing bacteria.Microb Cell Fact. 2023 Jun 9;22(1):111. doi: 10.1186/s12934-023-02120-3. Microb Cell Fact. 2023. PMID: 37296442 Free PMC article.
-
Metabolic engineering of Lactobacilli spp. for disease treatment.Microb Cell Fact. 2025 Mar 6;24(1):53. doi: 10.1186/s12934-025-02682-4. Microb Cell Fact. 2025. PMID: 40050843 Free PMC article. Review.
-
Modulatory Effects of Probiotics During Pathogenic Infections With Emphasis on Immune Regulation.Front Immunol. 2021 Apr 8;12:616713. doi: 10.3389/fimmu.2021.616713. eCollection 2021. Front Immunol. 2021. PMID: 33897683 Free PMC article. Review.
-
Immune Evaluation of Recombinant Lactobacillus plantarum With Surface Display of HA1-DCpep in Mice.Front Immunol. 2021 Dec 1;12:800965. doi: 10.3389/fimmu.2021.800965. eCollection 2021. Front Immunol. 2021. PMID: 34925386 Free PMC article.
References
-
- Yang G, Yao J, Yang W, Jiang Y, Du J, Huang H, Gu W, Hu J, Ye L, Shi C, et al. Construction and immunological evaluation of recombinant Lactobacillus plantarum expressing SO7 of Eimeria tenella fusion DC-targeting peptide. Vet Parasitol. 2017;236:7–13. doi: 10.1016/j.vetpar.2017.01.023. - DOI - PubMed
-
- Liu L, Zhang W, Song Y, Wang W, Zhang Y, Wang T, Li K, Pan Q, Qi X, Gao Y, et al. Recombinant Lactococcus lactis co-expressing OmpH of an M cell-targeting ligand and IBDV-VP2 protein provide immunological protection in chickens. Vaccine. 2018;36:729–735. doi: 10.1016/j.vaccine.2017.12.027. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2017YFD0501200/National Key Research and Development Program of China
- 2017YFD0501000/National Key Research and Development Program of China
- 2017YFD0500400/National Key Research and Development Program of China
- 31672528/National Natural Science Foundation of China
- 31602092/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

