Immune response in piglets orally immunized with recombinant Bacillus subtilis expressing the capsid protein of porcine circovirus type 2
- PMID: 32046726
- PMCID: PMC7014726
- DOI: 10.1186/s12964-020-0514-4
Immune response in piglets orally immunized with recombinant Bacillus subtilis expressing the capsid protein of porcine circovirus type 2
Abstract
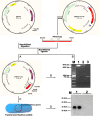
Background: Porcine circovirus type 2 (PCV2) is the causative agent of postweaning multisystemic wasting syndrome, and is associated with a number of other diseases. PCV2 is widely distributed in most developed swine industries, and is a severe economic burden. With an eye to developing an effective, safe, and convenient vaccine against PCV2-associated diseases, we have constructed a recombinant Bacillus subtilis strain (B. subtilis-Cap) that expresses the PCV2 capsid protein (Cap).
Methods: Electroporation of a plasmid shuttle vector encoding the PCV2 Cap sequence was use to transform Bacillus subtilis. Flow cytometry was used to evaluate in vitro bone marrow derived dendritic cell (BM-DC) maturation and T cell proliferation induced by B. subtilis-Cap. Orally inoculated piglets were used for in vivo experiments; ELISA and western blotting were used to evaluate B. subtilis-Cap induced PCV2-specific IgA and IgG levels, as well as the secretion of cytokines and the expression of Toll-like receptor 2 (TLR2) and Toll-like receptor 9 (TLR9).
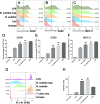
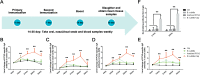
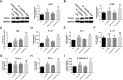
Results: We evaluated the immune response to B. subtilis-Cap in vitro using mouse BM-DCs and in vivo using neonatal piglets orally inoculated with B. subtilis-Cap. Our results showed that the recombinant B. subtilis-Cap activated BM-DCs, significantly increased co-stimulatory molecules (CD40 and CD80) and major histocompatibility complex II, and induced allogenic T cells proliferation. Piglets immunized with B. subtilis-Cap had elevated levels of PCV2-specific IgA in the mucosal tissues of the digestive and respiratory tract, and PCV2-specific IgG in serum (P < 0.05 or P < 0.01). Ileal immunocompetent cells, such as the IgA-secreting cells (P < 0.01), intestinal intraepithelial lymphocytes (IELs) (P < 0.01), CD3+ T lymphocytes (P < 0.01) and CD4+ T lymphocytes (P < 0.01) increased significantly in the B. subtilis-Cap immunized piglets. Additionally, B. subtilis-Cap inoculation resulted in increased the expression of TLR2 and TLR9 (P < 0.01), and induced the secretion of cytokines IL-1β, IL-6, interferon-γ, and β-defensin 2 (P < 0.01).
Conclusions: We constructed a prototype PCV2 vaccine that can be administered orally and elicits a more robust humoral and cellular immunity than inactivated PCV2. B. subtilis-Cap is a promising vaccine candidate that is safe, convenient, and inexpensive. Further in vivo research is needed to determine its full range of efficacy in pigs.
Keywords: Bacillus subtilis WB800N; Capsid protein; Dendritic cells; Immune responses; Neonatal piglets; Porcine circovirus type 2 (PCV2).
Conflict of interest statement
The authors declare they have no competing interests.
Figures

References
-
- Nawagitgul P, Morozov I, Bolin SR, Harms PA, Sorden SD, Paul PS. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. Jgenvirol. 2000;81:2281–2287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

