Presenilin1 exerts antiproliferative effects by repressing the Wnt/β-catenin pathway in glioblastoma
- PMID: 32046730
- PMCID: PMC7014622
- DOI: 10.1186/s12964-019-0501-9
Presenilin1 exerts antiproliferative effects by repressing the Wnt/β-catenin pathway in glioblastoma
Abstract
Background: Glioblastoma and Alzheimer's disease (AD) are the most common and devastating diseases in the central nervous system. The dysfunction of Presenilin1 is the main reason for AD pathogenesis. However, the molecular function of Presenilin1 and its relative mechanism in glioblastoma remain unclear.
Methods: Expression of presenilin1 in glioma was determined by IHC. CCK-8, colony formation, Flow cytometry, Edu staining were utilized to evaluate functions of presenilin1 on glioblastoma proliferation. The mechanism of above process was assessed by Western blotting and cell immunofluorescence. Mouse transplanting glioblastoma model and micro-MRI detection were used to verified presenilin1 function in vivo.
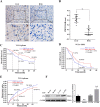
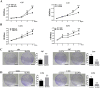
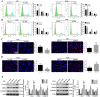
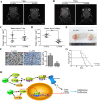
Results: In this study, we found that all grades of glioma maintained relatively low Presenilin1 expression and that the expression of Presenilin1 in high-grade glioma was significantly lower than that in low-grade glioma. Moreover, the Presenilin1 level had a positive correlation with glioma and glioblastoma patient prognosis. Next, we determined that Presenilin1 inhibited the growth and proliferation of glioblastoma cells by downregulating CDK6, C-myc and Cyclin D1 to arrest the cell cycle at the G1/S phase. Mechanistically, Presenilin1 promoted the direct phosphorylation of β-catenin at the 45 site and indirect phosphorylation at the 33/37/41 site, then decreased the stabilized part of β-catenin and hindered its translocation from the cytoplasm to the nucleus. Furthermore, we found that Presenilin1 downregulation clearly accelerated the growth of subcutaneous glioblastoma, and Presenilin1 overexpression significantly repressed the subcutaneous and intracranial transplantation of glioblastoma by hindering β-catenin-dependent cell proliferation.
Conclusion: Our data implicate the antiproliferative effect of Presenilin1 in glioblastoma by suppressing Wnt/β-catenin signaling, which may provide a novel therapeutic agent for glioblastoma. Video Abstract.
Keywords: Cell cycle; Glioblastoma; Presenilin1; Proliferation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Presenilin1 inhibits glioblastoma cell invasiveness via promoting Sortilin cleavage.Cell Commun Signal. 2021 Nov 15;19(1):112. doi: 10.1186/s12964-021-00780-5. Cell Commun Signal. 2021. PMID: 34781973 Free PMC article.
-
Deubiquitinase USP9X deubiquitinates β-catenin and promotes high grade glioma cell growth.Oncotarget. 2016 Nov 29;7(48):79515-79525. doi: 10.18632/oncotarget.12819. Oncotarget. 2016. PMID: 27783990 Free PMC article.
-
Long noncoding RNA OIP5-AS1 targets Wnt-7b to affect glioma progression via modulation of miR-410.Biosci Rep. 2019 Jan 3;39(1):BSR20180395. doi: 10.1042/BSR20180395. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30498093 Free PMC article.
-
miR-504 suppresses mesenchymal phenotype of glioblastoma by directly targeting the FZD7-mediated Wnt-β-catenin pathway.J Exp Clin Cancer Res. 2019 Aug 16;38(1):358. doi: 10.1186/s13046-019-1370-1. J Exp Clin Cancer Res. 2019. PMID: 31419987 Free PMC article.
-
WNT Signaling as a Therapeutic Target for Glioblastoma.Int J Mol Sci. 2021 Aug 5;22(16):8428. doi: 10.3390/ijms22168428. Int J Mol Sci. 2021. PMID: 34445128 Free PMC article. Review.
Cited by
-
MicroRNAs: pioneering regulators in Alzheimer's disease pathogenesis, diagnosis, and therapy.Transl Psychiatry. 2024 Sep 10;14(1):367. doi: 10.1038/s41398-024-03075-8. Transl Psychiatry. 2024. PMID: 39256358 Free PMC article. Review.
-
The critical role of γ-secretase and its inhibitors in cancer and cancer therapeutics.Int J Biol Sci. 2023 Oct 2;19(16):5089-5103. doi: 10.7150/ijbs.87334. eCollection 2023. Int J Biol Sci. 2023. PMID: 37928268 Free PMC article. Review.
-
Estimation of genotoxicity, apoptosis and oxidative stress induction by TiO2 nanoparticles and acrylamide subacute oral coadministration in mice.Sci Rep. 2022 Nov 4;12(1):18648. doi: 10.1038/s41598-022-23302-w. Sci Rep. 2022. PMID: 36333451 Free PMC article.
-
Yttrium oxide nanoparticles ameliorates calcium hydroxide and calcium titanate nanoparticles induced genomic DNA and mitochondrial damage, ROS generation and inflammation.Sci Rep. 2024 Jun 6;14(1):13015. doi: 10.1038/s41598-024-62877-4. Sci Rep. 2024. PMID: 38844752 Free PMC article.
-
Presenilin-1 (PSEN1) Mutations: Clinical Phenotypes beyond Alzheimer's Disease.Int J Mol Sci. 2023 May 8;24(9):8417. doi: 10.3390/ijms24098417. Int J Mol Sci. 2023. PMID: 37176125 Free PMC article. Review.
References
-
- Aldape Kenneth, Brindle Kevin M., Chesler Louis, Chopra Rajesh, Gajjar Amar, Gilbert Mark R., Gottardo Nicholas, Gutmann David H., Hargrave Darren, Holland Eric C., Jones David T. W., Joyce Johanna A., Kearns Pamela, Kieran Mark W., Mellinghoff Ingo K., Merchant Melinda, Pfister Stefan M., Pollard Steven M., Ramaswamy Vijay, Rich Jeremy N., Robinson Giles W., Rowitch David H., Sampson John H., Taylor Michael D., Workman Paul, Gilbertson Richard J. Challenges to curing primary brain tumours. Nature Reviews Clinical Oncology. 2019;16(8):509–520. doi: 10.1038/s41571-019-0177-5. - DOI - PMC - PubMed
-
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, Et A. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

