Heterologous caffeic acid biosynthesis in Escherichia coli is affected by choice of tyrosine ammonia lyase and redox partners for bacterial Cytochrome P450
- PMID: 32046741
- PMCID: PMC7011507
- DOI: 10.1186/s12934-020-01300-9
Heterologous caffeic acid biosynthesis in Escherichia coli is affected by choice of tyrosine ammonia lyase and redox partners for bacterial Cytochrome P450
Abstract
Background: Caffeic acid is industrially recognized for its antioxidant activity and therefore its potential to be used as an anti-inflammatory, anticancer, antiviral, antidiabetic and antidepressive agent. It is traditionally isolated from lignified plant material under energy-intensive and harsh chemical extraction conditions. However, over the last decade bottom-up biosynthesis approaches in microbial cell factories have been established, that have the potential to allow for a more tailored and sustainable production. One of these approaches has been implemented in Escherichia coli and only requires a two-step conversion of supplemented L-tyrosine by the actions of a tyrosine ammonia lyase and a bacterial Cytochrome P450 monooxygenase. Although the feeding of intermediates demonstrated the great potential of this combination of heterologous enzymes compared to others, no de novo synthesis of caffeic acid from glucose has been achieved utilizing the bacterial Cytochrome P450 thus far.
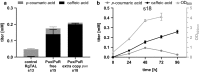
Results: The herein described work aimed at improving the efficiency of this two-step conversion in order to establish de novo caffeic acid formation from glucose. We implemented alternative tyrosine ammonia lyases that were reported to display superior substrate binding affinity and selectivity, and increased the efficiency of the Cytochrome P450 by altering the electron-donating redox system. With this strategy we were able to achieve final titers of more than 300 µM or 47 mg/L caffeic acid over 96 h in an otherwise wild type E. coli MG1655(DE3) strain with glucose as the only carbon source. We observed that the choice and gene dose of the redox system strongly influenced the Cytochrome P450 catalysis. In addition, we were successful in applying a tethering strategy that rendered even a virtually unproductive Cytochrome P450/redox system combination productive.
Conclusions: The caffeic acid titer achieved in this study is about 10% higher than titers reported for other heterologous caffeic acid pathways in wildtype E. coli without L-tyrosine supplementation. The tethering strategy applied to the Cytochrome P450 appears to be particularly useful for non-natural Cytochrome P450/redox partner combinations and could be useful for other recombinant pathways utilizing bacterial Cytochromes P450.
Keywords: Caffeic acid; Cytochrome P450; PUPPET; Recombinant pathway; Tethering.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Heterologous production of caffeic acid from tyrosine in Escherichia coli.Enzyme Microb Technol. 2015 Apr;71:36-44. doi: 10.1016/j.enzmictec.2015.01.001. Epub 2015 Jan 14. Enzyme Microb Technol. 2015. PMID: 25765308
-
Biosynthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex.Microb Cell Fact. 2012 Apr 4;11:42. doi: 10.1186/1475-2859-11-42. Microb Cell Fact. 2012. PMID: 22475509 Free PMC article.
-
De novo Biosynthesis of Caffeic Acid and Chlorogenic Acid in Escherichia coli via Enzyme Engineering and Pathway Engineering.ACS Synth Biol. 2025 May 16;14(5):1581-1593. doi: 10.1021/acssynbio.4c00850. Epub 2025 Apr 15. ACS Synth Biol. 2025. PMID: 40232288
-
P450BM-3; a tale of two domains--or is it three?Steroids. 1997 Jan;62(1):117-23. doi: 10.1016/s0039-128x(96)00169-9. Steroids. 1997. PMID: 9029725 Review.
-
Development of a combined biological and chemical process for production of industrial aromatics from renewable resources.Annu Rev Microbiol. 2007;61:51-69. doi: 10.1146/annurev.micro.61.080706.093248. Annu Rev Microbiol. 2007. PMID: 17456010 Review.
Cited by
-
Synthetic Scaffold Systems for Increasing the Efficiency of Metabolic Pathways in Microorganisms.Biology (Basel). 2021 Mar 11;10(3):216. doi: 10.3390/biology10030216. Biology (Basel). 2021. PMID: 33799683 Free PMC article. Review.
-
Heterologous production of caffeic acid in microbial hosts: current status and perspectives.Front Microbiol. 2025 Apr 29;16:1570406. doi: 10.3389/fmicb.2025.1570406. eCollection 2025. Front Microbiol. 2025. PMID: 40365059 Free PMC article. Review.
-
Ferulic acid production by metabolically engineered Escherichia coli.Bioresour Bioprocess. 2021 Aug 10;8(1):70. doi: 10.1186/s40643-021-00423-0. Bioresour Bioprocess. 2021. PMID: 38650224 Free PMC article.
-
Enabling Aromatic Hydroxylation in a Cytochrome P450 Monooxygenase Enzyme through Protein Engineering.Chemistry. 2022 Dec 1;28(67):e202201895. doi: 10.1002/chem.202201895. Epub 2022 Oct 6. Chemistry. 2022. PMID: 36043399 Free PMC article.
-
A targeted metabolomics method for extra- and intracellular metabolite quantification covering the complete monolignol and lignan synthesis pathway.Metab Eng Commun. 2022 Aug 31;15:e00205. doi: 10.1016/j.mec.2022.e00205. eCollection 2022 Dec. Metab Eng Commun. 2022. PMID: 36119807 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

