Aquaporin 1 and the Na+/K+/2Cl- cotransporter 1 are present in the leptomeningeal vasculature of the adult rodent central nervous system
- PMID: 32046744
- PMCID: PMC7014736
- DOI: 10.1186/s12987-020-0176-z
Aquaporin 1 and the Na+/K+/2Cl- cotransporter 1 are present in the leptomeningeal vasculature of the adult rodent central nervous system
Abstract
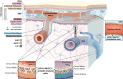
Background: The classical view of cerebrospinal fluid (CSF) production posits the choroid plexus as its major source. Although previous studies indicate that part of CSF production occurs in the subarachnoid space (SAS), the mechanisms underlying extra-choroidal CSF production remain elusive. We here investigated the distributions of aquaporin 1 (AQP1) and Na+/K+/2Cl- cotransporter 1 (NKCC1), key proteins for choroidal CSF production, in the adult rodent brain and spinal cord.
Methods: We have accessed AQP1 distribution in the intact brain using uDISCO tissue clearing technique and by Western blot. AQP1 and NKCC1 cellular localization were accessed by immunohistochemistry in brain and spinal cord obtained from adult rodents. Imaging was performed using light-sheet, confocal and bright field light microscopy.
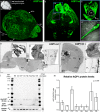
Results: We determined that AQP1 is widely distributed in the leptomeningeal vasculature of the intact brain and that its glycosylated isoform is the most prominent in different brain regions. Moreover, AQP1 and NKCC1 show specific distributions in the smooth muscle cell layer of penetrating arterioles and veins in the brain and spinal cord, and in the endothelia of capillaries and venules, restricted to the SAS vasculature.
Conclusions: Our results shed light on the molecular framework that may underlie extra-choroidal CSF production and we propose that AQP1 and NKCC1 within the leptomeningeal vasculature, specifically at the capillary level, are poised to play a role in CSF production throughout the central nervous system.
Keywords: Aquaporin 1; Capillaries; Leptomeningeal vasculature; NKCC1; Penetrating arterioles; Subarachnoid space; Veins; Venules.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
The Na+,K+,2Cl- Cotransporter, Not Aquaporin 1, Sustains Cerebrospinal Fluid Secretion While Controlling Brain K+ Homeostasis.Adv Sci (Weinh). 2025 Feb;12(6):e2409120. doi: 10.1002/advs.202409120. Epub 2024 Dec 18. Adv Sci (Weinh). 2025. PMID: 39692709 Free PMC article.
-
Increased aquaporin-1 and Na+ -K+ -2Cl- cotransporter 1 expression in choroid plexus leads to blood-cerebrospinal fluid barrier disruption and necrosis of hippocampal CA1 cells in acute rat models of hyponatremia.J Neurosci Res. 2012 Jul;90(7):1437-44. doi: 10.1002/jnr.23017. Epub 2012 Mar 15. J Neurosci Res. 2012. PMID: 22419034
-
Cotransporter-mediated water transport underlying cerebrospinal fluid formation.Nat Commun. 2018 Jun 4;9(1):2167. doi: 10.1038/s41467-018-04677-9. Nat Commun. 2018. PMID: 29867199 Free PMC article.
-
Hydrocephalus and aquaporins: the role of aquaporin-1.Acta Neurochir Suppl. 2012;113:51-4. doi: 10.1007/978-3-7091-0923-6_11. Acta Neurochir Suppl. 2012. PMID: 22116423 Review.
-
Water and solute secretion by the choroid plexus.Pflugers Arch. 2007 Apr;454(1):1-18. doi: 10.1007/s00424-006-0170-6. Epub 2006 Nov 21. Pflugers Arch. 2007. PMID: 17120021 Review.
Cited by
-
Choroid Plexus Aquaporins in CSF Homeostasis and the Glymphatic System: Their Relevance for Alzheimer's Disease.Int J Mol Sci. 2023 Jan 3;24(1):878. doi: 10.3390/ijms24010878. Int J Mol Sci. 2023. PMID: 36614315 Free PMC article. Review.
-
Non-Aquaporin Water Channels.Adv Exp Med Biol. 2023;1398:331-342. doi: 10.1007/978-981-19-7415-1_23. Adv Exp Med Biol. 2023. PMID: 36717505
-
Physiology of Glymphatic Solute Transport and Waste Clearance from the Brain.Physiology (Bethesda). 2022 Nov 1;37(6):0. doi: 10.1152/physiol.00015.2022. Epub 2022 Jul 26. Physiology (Bethesda). 2022. PMID: 35881783 Free PMC article. Review.
-
Choroid plexus-targeted NKCC1 overexpression to treat post-hemorrhagic hydrocephalus.Neuron. 2023 May 17;111(10):1591-1608.e4. doi: 10.1016/j.neuron.2023.02.020. Epub 2023 Mar 8. Neuron. 2023. PMID: 36893755 Free PMC article.
-
The glymphatic system: implications for drugs for central nervous system diseases.Nat Rev Drug Discov. 2022 Oct;21(10):763-779. doi: 10.1038/s41573-022-00500-9. Epub 2022 Aug 10. Nat Rev Drug Discov. 2022. PMID: 35948785 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

