Evaluation of the effects of pycnogenol (French maritime pine bark extract) supplementation on inflammatory biomarkers and nutritional and clinical status in traumatic brain injury patients in an intensive care unit: A randomized clinical trial protocol
- PMID: 32046747
- PMCID: PMC7014642
- DOI: 10.1186/s13063-019-4008-x
Evaluation of the effects of pycnogenol (French maritime pine bark extract) supplementation on inflammatory biomarkers and nutritional and clinical status in traumatic brain injury patients in an intensive care unit: A randomized clinical trial protocol
Abstract
Background: Traumatic brain injury (TBI) is one of the major health and socioeconomic problems in the world. Immune-enhancing enteral formula has been proven to significantly reduce infection rate in TBI patients. One of the ingredients that can be used in immunonutrition formulas to reduce inflammation and oxidative stress is pycnogenol.
Objective: The objective of this work is to survey the effect of pycnogenol on the clinical, nutritional, and inflammatory status of TBI patients.
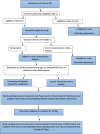
Methods: This is a double-blind, randomized controlled trial. Block randomization will be used. An intervention group will receive pycnogenol supplementation of 150 mg for 10 days and a control group will receive a placebo for the same duration. Inflammatory status (IL-6, IL- 1β, C-reactive protein) and oxidative stress status (malondialdehyde, total antioxidant capacity), at the baseline, at the 5th day, and at the end of the study (10th day) will be measured. Clinical and nutritional status will be assessed three times during the intervention. The Sequential Organ Failure Assessment (SOFA) questionnaire for assessment of organ failure will be filled out every other day. The mortality rate will be calculated within 28 days of the start of the intervention. Weight, body mass index, and body composition will be measured. All analyses will be conducted by an initially assigned study arm in an intention-to-treat analysis.
Discussion: We expect that supplementation of 150 mg pycnogenol for 10 days will improve clinical and nutritional status and reduce the inflammation and oxidative stress of the TBI patients.
Trial registration: This trial is registered at clinicaltrials.gov (ref: NCT03777683) at 12/13/2018.
Keywords: Critical care; French maritime pine bark extract; Inflammation; Nutrition support; Pycnogenol; Traumatic brain injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Williams AL. Traumatic brain injury. Physical Management for Neurological Conditions E-Book. 2018.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials